Katie Kobara, Steve Bleyl, Alan Rope, Jacob E. Berchuck, Jamie Esposito, Colleen Caleshu, Scott M. Weissman
Context, follow-up, and diagnostic outcomes of patients who have a cancer signal detected from a multi-cancer early detection test (CAN92)
Context, follow-up, and diagnostic outcomes of patients who have a cancer signal detected from a multi-cancer early detection test (CAN92)
Katie Kobara, Steve Bleyl, Alan Rope, Jacob E. Berchuck, Jamie Esposito, Colleen Caleshu, Scott M. Weissman
Background
A multi-cancer early detection (MCED) test became commercially available in 2021, and is intended for use in patients at elevated risk for cancer. This test can identify multiple cancer types through detection of circulating tumor DNA in blood. Little is known about the experiences of patients who receive a cancer signal detected, which is a result suggestive of the presence of cancer.
Purpose
We investigated symptomatic context, access to follow-up care, and diagnostic outcome of early-adopters of MCED testing who received a cancer signal detected.
Methods
We performed a chart review of consecutive patients with a cancer signal detected from MCED testing who were seen in our telehealth genetics practice from June 2021 to April 2022. We extracted age, sex, MCED result, nature of follow-up care, and diagnostic outcome.
Results
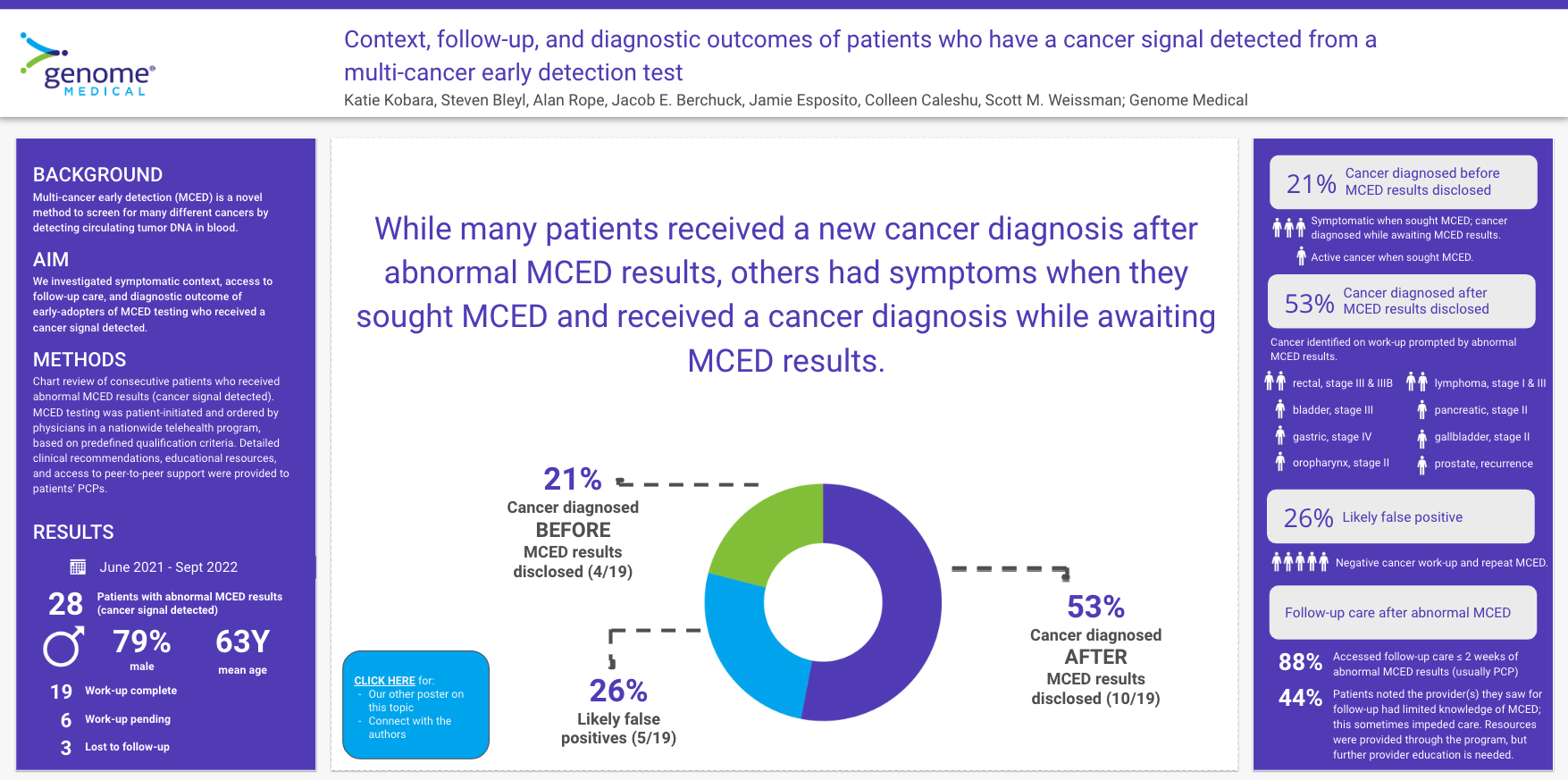
There were 18 patients with a cancer signal detected; mean age was 63 years (SD 8.7) and the majority were male (78%; 14/18). These patients fell into four subgroups. First, patients who had an active cancer diagnosis at the time of results disclosure (22%; 4/18). Most (75%; 3/4) were experiencing symptoms but did not have a cancer diagnosis at the time they requested MCED testing, with the one remaining patient having a pre-existing active cancer diagnosis. Second, patients who received a new cancer diagnosis prompted by the MCED result (28%; 5/18), in which the following cancers were diagnosed: colorectal cancer (n=2; both stage III), gastric (n=1; stage IV), oropharynx (n=1; stage II), and bladder (n=1; stage III). In this group, one patient was experiencing symptoms at the time they requested MCED testing while the remaining were asymptomatic. Third, patients with no cancer diagnosed on clinical follow-up (22%; 4/18), and fourth, patients still undergoing evaluation for cancer (28%; 5/18). All patients in groups 2 and 3 (9/9) accessed follow-up care within two weeks of results disclosure, most (78%; 7/9) with their primary care provider. The main challenge patients identified was limited provider knowledge about MCED testing (67%; 6/9), which sometimes obstructed or delayed subsequent diagnostic evaluation (67%; 4/6).
Conclusion
MCED testing led to new cancer diagnoses in 28% (5/18). Some patients had symptoms which prompted them to seek MCED testing; research is needed to understand patient motivations for pursuing this testing. Patients were able to access an initial follow-up appointment in a timely fashion. More education on MCED testing will be important for widespread uptake.
Background
A multi-cancer early detection (MCED) test became commercially available in 2021, and is intended for use in patients at elevated risk for cancer. This test can identify multiple cancer types through detection of circulating tumor DNA in blood. Little is known about the experiences of patients who receive a cancer signal detected, which is a result suggestive of the presence of cancer.
Purpose
We investigated symptomatic context, access to follow-up care, and diagnostic outcome of early-adopters of MCED testing who received a cancer signal detected.
Methods
We performed a chart review of consecutive patients with a cancer signal detected from MCED testing who were seen in our telehealth genetics practice from June 2021 to April 2022. We extracted age, sex, MCED result, nature of follow-up care, and diagnostic outcome.
Results
There were 18 patients with a cancer signal detected; mean age was 63 years (SD 8.7) and the majority were male (78%; 14/18). These patients fell into four subgroups. First, patients who had an active cancer diagnosis at the time of results disclosure (22%; 4/18). Most (75%; 3/4) were experiencing symptoms but did not have a cancer diagnosis at the time they requested MCED testing, with the one remaining patient having a pre-existing active cancer diagnosis. Second, patients who received a new cancer diagnosis prompted by the MCED result (28%; 5/18), in which the following cancers were diagnosed: colorectal cancer (n=2; both stage III), gastric (n=1; stage IV), oropharynx (n=1; stage II), and bladder (n=1; stage III). In this group, one patient was experiencing symptoms at the time they requested MCED testing while the remaining were asymptomatic. Third, patients with no cancer diagnosed on clinical follow-up (22%; 4/18), and fourth, patients still undergoing evaluation for cancer (28%; 5/18). All patients in groups 2 and 3 (9/9) accessed follow-up care within two weeks of results disclosure, most (78%; 7/9) with their primary care provider. The main challenge patients identified was limited provider knowledge about MCED testing (67%; 6/9), which sometimes obstructed or delayed subsequent diagnostic evaluation (67%; 4/6).
Conclusion
MCED testing led to new cancer diagnoses in 28% (5/18). Some patients had symptoms which prompted them to seek MCED testing; research is needed to understand patient motivations for pursuing this testing. Patients were able to access an initial follow-up appointment in a timely fashion. More education on MCED testing will be important for widespread uptake.


