What if We Found Cancer Early Enough to Make a Difference
The Galleri® multi-cancer early detection test detects a cancer signal across more than 50 types of cancer.1,2 It’s now available to eligible Illumina employees and their dependents.
Individuals must meet the clinical eligibility criteria determined by Genome Medical to receive the test.
What if We Found Cancer Early Enough to Make a Difference
The Galleri® multi-cancer early detection test detects a cancer signal across more than 50 types of cancer.1,2 It’s now available to eligible Illumina employees and their dependents.
Individuals must meet the clinical eligibility criteria determined by Genome Medical to receive the test.
Finding Cancer Early is Important
Thinking about the possibility of having cancer can be overwhelming, but taking steps to find cancer early can help you feel more in control. Often, the earlier that cancer can be found, the higher the chance of better outcomes.3
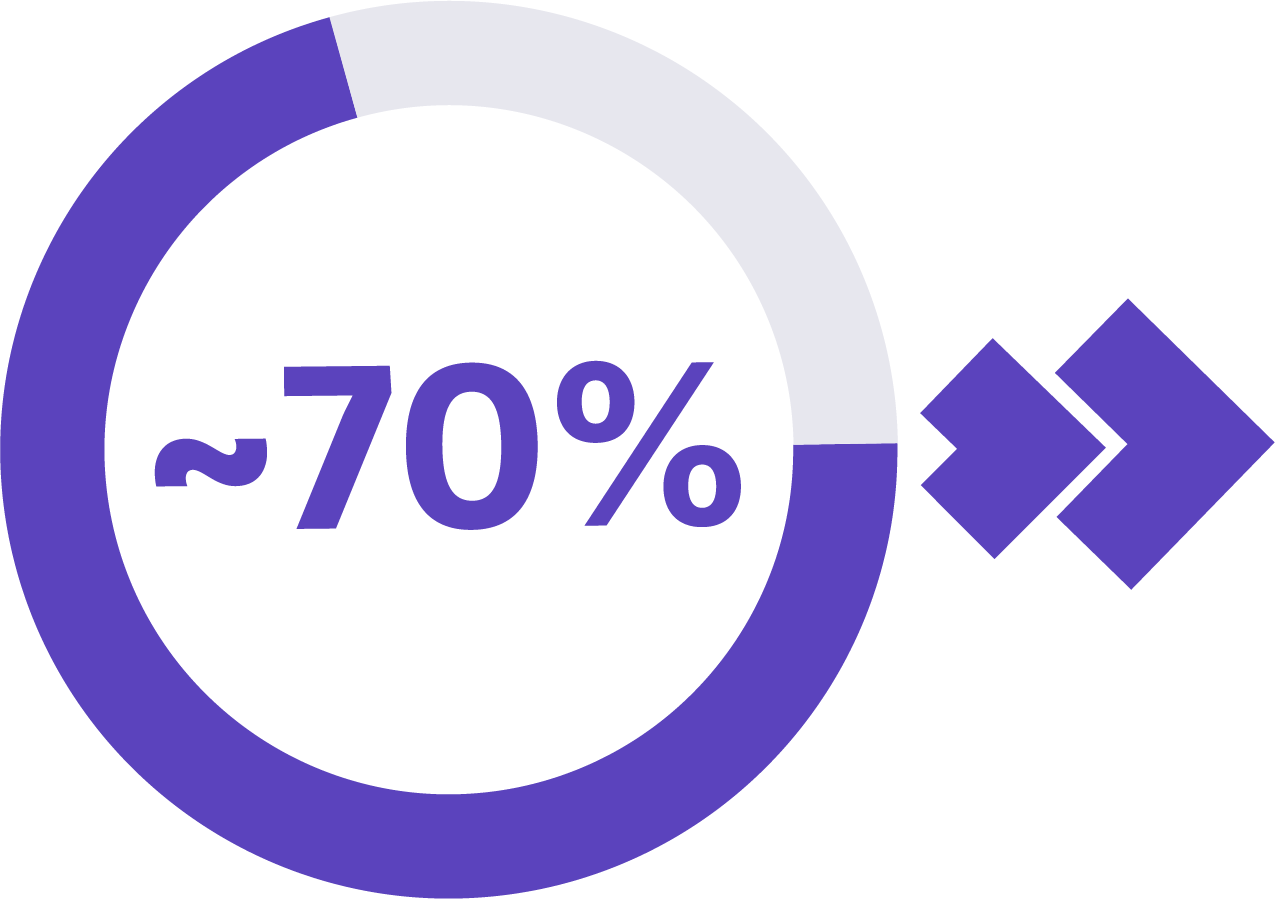
of cancer deaths in people ages 50-79 are caused by cancers not commonly screened for.4,5,6
In fact, when cancers are diagnosed early before they have had a chance to spread, the overall 5-year survival rate is 4x higher than when diagnosed in later stages.3,7
Finding Cancer Early is Important
Thinking about the possibility of having cancer can be overwhelming, but taking steps to find cancer early can help you feel more in control. Often, the earlier that cancer can be found, the higher the chance of better outcomes.3
of cancer deaths in people ages 50-79 are caused by cancers not commonly screened for.4,5,6
In fact, when cancers are diagnosed early before they have had a chance to spread, the overall 5-year survival rate is 4x higher than when diagnosed in later stages.3,7
*Video embedded from YouTube
The Galleri test, GRAIL’s multi-cancer early detection test, detects a cancer signal across more than 50 types of cancer, many of which are not commonly screened for today. All that’s required is a simple blood draw.1,2,8
Benefit Requirements & Eligibility
This employee benefit through Illumina and Genome Medical provides eligible employees access to the Galleri multi-cancer early detection test as an annual benefit. Annual eligibility is based on a rolling calendar year from when your test results were completed and reported to you. If you received Galleri test results in the past, Genome Medical will notify you once you’re eligible to request the test again.
Genome Medical’s team of oncology experts has reviewed risk factors that increase a person’s chance to develop cancer to determine who would benefit from the Galleri test. The current criteria are not meant to represent a comprehensive list of all cancer risk factors but are a starting point to offer the Galleri test.
Between the ages of 22-49?
Certain risk factors may put you at an elevated risk for developing cancer. The Galleri test may be right for you if you meet one or more of the following criteria:
- You have a history of cancer (excluding basal or squamous cell carcinomas of the skin) and are more than three years out of treatment. A consultation with a genetic counselor is required if you have had a personal history of cancer and are more than three years out of treatment.
- You have a first degree relative (parent, child and/or full sibling) who has had cancer
- You are a smoker or have quit smoking in the last 10 years
- You had previous genetic testing and were found to have a gene mutation confirming an increased cancer risk due to a hereditary cancer syndrome (e.g., BRCA1/2, Lynch syndrome, CHEK2). Please note that a clinical report from an accredited laboratory that performs clinical grade genetic testing is required; this excludes tests from 23andMe, Ancestry.com and other direct to consumer genetic testing companies. A consultation with a genetic counselor is required if you have had a previous gene mutation in order to confirm the finding.
50 OR OLDER?
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those aged 50 or older. The risk of cancer increases as we age, so individuals 50 or older are eligible for the Galleri Test.
CURRENTLY, THE FOLLOWING INDIVIDUALS ARE NOT ELIGIBLE FOR THE GALLERI TEST
- You have been diagnosed or treated for cancer within the past three years
- This excludes basal or squamous cell carcinomas of the skin
- This also excludes hormonal therapy (e.g., Tamoxifen, Arimidex, Aromasin) for breast cancer; you need to be 3 years past surgery, chemotherapy and/or radiotherapy but can still be taking hormonal therapy
- You are currently pregnant
- You are 21 years old or younger
- You live outside of the United States; Genome Medical is not able to order testing for people who reside outside the US
Benefit Requirements & Eligibility
This employee benefit through Illumina and Genome Medical provides eligible employees access to the Galleri multi-cancer early detection test as an annual benefit. Annual eligibility is based on a rolling calendar year from when your test results were completed and reported to you. If you received Galleri test results in the past, Genome Medical will notify you once you’re eligible to request the test again.
Genome Medical’s team of oncology experts has reviewed risk factors that increase a person’s chance to develop cancer to determine who would benefit from the Galleri test. The current criteria are not meant to represent a comprehensive list of all cancer risk factors but are a starting point to offer the Galleri test.
BETWEEN THE AGES OF 22-49?
Certain risk factors may put you at an elevated risk for developing cancer. The Galleri test may be right for you if you meet one or more of the following criteria:
- You have a history of cancer (excluding basal or squamous cell carcinomas of the skin) and are more than three years out of treatment. A consultation with a genetic counselor is required if you have had a personal history of cancer and are more than three years out of treatment.
- You have a first degree relative (parent, child and/or full sibling) who has had cancer
- You are a smoker or have quit smoking in the last 10 years
- You had previous genetic testing and were found to have a gene mutation confirming an increased cancer risk due to a hereditary cancer syndrome (e.g., BRCA1/2, Lynch syndrome, CHEK2). Please note that a clinical report from an accredited laboratory that performs clinical grade genetic testing is required; this excludes tests from 23andMe, Ancestry.com and other direct to consumer genetic testing companies. A consultation with a genetic counselor is required if you have had a previous gene mutation in order to confirm the finding.
50 OR OLDER?
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those aged 50 or older. The risk of cancer increases as we age, so individuals 50 or older are eligible for the Galleri Test.
CURRENTLY, THE FOLLOWING INDIVIDUALS ARE NOT ELIGIBLE FOR THE GALLERI TEST
- You have been diagnosed or treated for cancer within the past three years
- This excludes basal or squamous cell carcinomas of the skin
- This also excludes hormonal therapy (e.g., Tamoxifen, Arimidex, Aromasin) for breast cancer; you need to be 3 years past surgery, chemotherapy and/or radiotherapy but can still be taking hormonal therapy
- You are currently pregnant
- You are 21 years old or younger
- You live outside of the United States; Genome Medical is not able to order testing for people who reside outside the US
The Benefits of Multi-Cancer Detection
Early cancer detection
Detects many cancers not commonly screened for today, to allow for earlier treatment.1,8
Testing with ease
Completed with a simple blood draw.
Actionable results
If a cancer signal is found, the results can point to where in the body the cancer is coming from.
The Galleri test is intended to be used in addition to, and not replace, other cancer screening tests your health care provider recommends. The Galleri test does not detect all cancers nor does it measure your genetic risk of developing cancer in the future. The Galleri test is a screening test and does not detect all cancers. Further testing is needed to diagnose cancer if a signal is detected.
The Benefits of Multi-Cancer Detection
Early cancer detection
Detects many cancers not commonly screened for today, to allow for earlier treatment.1,8
Testing with ease
Completed with a simple blood draw.
Actionable results
If a cancer signal is found, the results can point to where in the body the cancer is coming from.
The Galleri test is intended to be used in addition to, and not replace, other cancer screening tests your health care provider recommends. The Galleri test does not detect all cancers nor does it measure your genetic risk of developing cancer in the future. The Galleri test is a screening test and does not detect all cancers. Further testing is needed to diagnose cancer if a signal is detected.
Test Process
Follow the process with just three steps.
Test Process
Follow the process with just three steps.
Ordering the Galleri test
The Galleri test must be ordered by a health care provider. If you’re ready to request the test, you’ll be asked to answer a few questions and a Genome Medical physician will review your information and determine if the test is right for you.
To learn more about clinical eligibility before requesting the test, please click here.

Ordering the
Galleri test
The Galleri test must be ordered by a health care provider. If you’re ready to request the test, you’ll be asked to answer a few questions and a Genome Medical physician will review your information and determine if the test is right for you.
To learn more about clinical eligibility before requesting the test, please click here.
Get a blood draw
If your test has been authorized by your Genome Medical physician, a Galleri collection kit will be mailed directly to you with directions on how to locate a partner laboratory to have your blood drawn. Please do not open the kit. You must take the kit to the lab unopened.
Make sure you have the following in hand for your blood draw:
Your Galleri collection kit
Will be shipped directly to you
Your completed and printed
Test Requisition Form
GRAIL will email this to you after your test has been authorized by Genome Medical
If you are a current patient who has questions about scheduling a blood draw, please find more information here or contact GRAIL Customer Service at 833-694-2553 or customerservice@grail.com.
Get a blood draw
If your test has been authorized by your Genome Medical physician, a Galleri collection kit will be mailed directly to you with directions on how to locate a partner laboratory to have your blood drawn. Please do not open the kit. You must take the kit to the lab unopened.
Make sure you have the following in hand for your blood draw:
Your Galleri collection kit
Will be shipped directly to you
Your completed and printed Test Requisition Form
GRAIL will email this to you after your test has been authorized by Genome Medical
If you are a current patient who has questions about scheduling a blood draw, please find more information here or contact GRAIL Customer Service at 833-694-2553 or customerservice@grail.com.
Receive your results and clinical action plan
Your results will be available approximately two weeks after your sample arrives at the GRAIL laboratory. At that time, Genome Medical will contact you via email or phone to review your test results and discuss next steps. Additionally, you’ll have the option to schedule a return of results session to further discuss your results. Once you receive your results, it’s important to share your results and clinical action plan with your primary care physician.
There are two possible results from the Galleri test:
No Cancer Signal Detected
This means that no cancer signal was found. However not all cancer types can be detected by the Galleri test, and the Galleri test may not detect all early stage cancers.
Cancer Signal Detected
This means that there is a suspicion of cancer. The Galleri test can point to where in the body (e.g., colon, head and neck) the cancer signal is coming from with high accuracy to help your health care provider guide next steps. Sometimes the test may indicate two locations in the body where the cancer signal may be coming from which may require separate follow-up evaluations.
Next steps:
Continue with all routine screening tests that your health care provider recommends. Missing routine cancer screenings or ignoring symptoms could lead to a delayed diagnosis of cancer. If you’re interested in repeating the Galleri test in one year, please contact Genome Medical.
Next steps:
The Galleri test does not diagnose cancer. Additional tests are needed to determine if cancer is present. Illumina has partnered with Included Health to connect you with health care providers, in addition to your primary care physician, who can order additional tests to determine if cancer is present. If you receive a cancer diagnosis, you have the option to work with Access Hope to ensure you have the support and resources you need.
The test does not measure your genetic risk of developing cancer in the future. With the Galleri multi-cancer early detection test, annual screening provides the opportunity to detect more cancers early.
False-positive and false-negative test results can occur. The Galleri test has a low false-positive rate of 0.5% (detecting a cancer signal when no cancer is present).1
Receive your results and clinical action plan
Your results will be available approximately two weeks after your sample arrives at the GRAIL laboratory. At that time, Genome Medical will contact you via email or phone to review your test results and discuss next steps. Additionally, you’ll have the option to schedule a return of results session to further discuss your results. Once you receive your results, it’s important to share your results and clinical action plan with your primary care physician.
There are two possible results from the Galleri test:
No Cancer Signal Detected
This means that no cancer signal was found. However not all cancer types can be detected by the Galleri test, and the Galleri test may not detect all early stage cancers.
Next steps:
Continue with all routine screening tests that your health care provider recommends. Missing routine cancer screenings or ignoring symptoms could lead to a delayed diagnosis of cancer. If you’re interested in repeating the Galleri test in one year, please contact Genome Medical.
Cancer Signal Detected
This means that there is a suspicion of cancer. The Galleri test can point to where in the body (e.g., colon, head and neck) the cancer signal is coming from with high accuracy to help your health care provider guide next steps. Sometimes the test may indicate two locations in the body where the cancer signal may be coming from which may require separate follow-up evaluations.
Next steps:
The Galleri test does not diagnose cancer. Additional tests are needed to determine if cancer is present. Illumina has partnered with Included Health to connect you with health care providers, in addition to your primary care physician, who can order additional tests to determine if cancer is present. If you receive a cancer diagnosis, you have the option to work with Access Hope to ensure you have the support and resources you need.
The test does not measure your genetic risk of developing cancer in the future. With the Galleri multi-cancer early detection test, annual screening provides the opportunity to detect more cancers early.
False-positive and false-negative test results can occur. The Galleri test has a low false-positive rate of 0.5% (detecting a cancer signal when no cancer is present).1
Breakthrough Test Performance
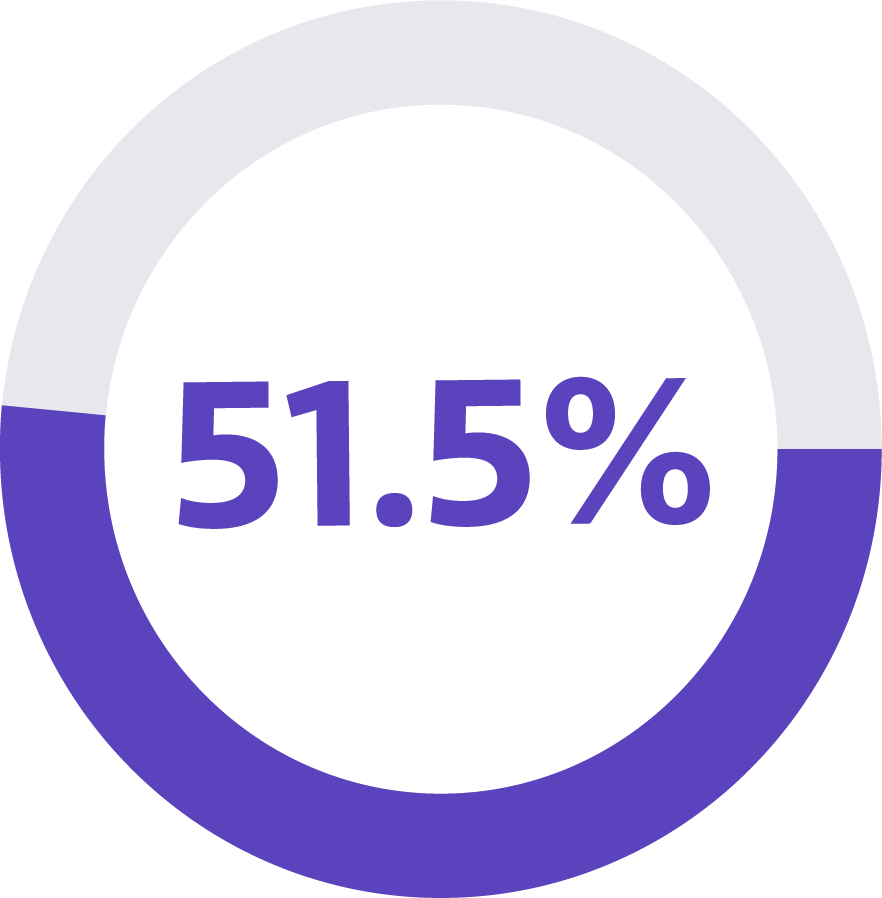
sensitivity*
This means that the Galleri test reported a “Cancer Signal Detected” result in approximately 52 individuals out of 100 who actually had cancer.1
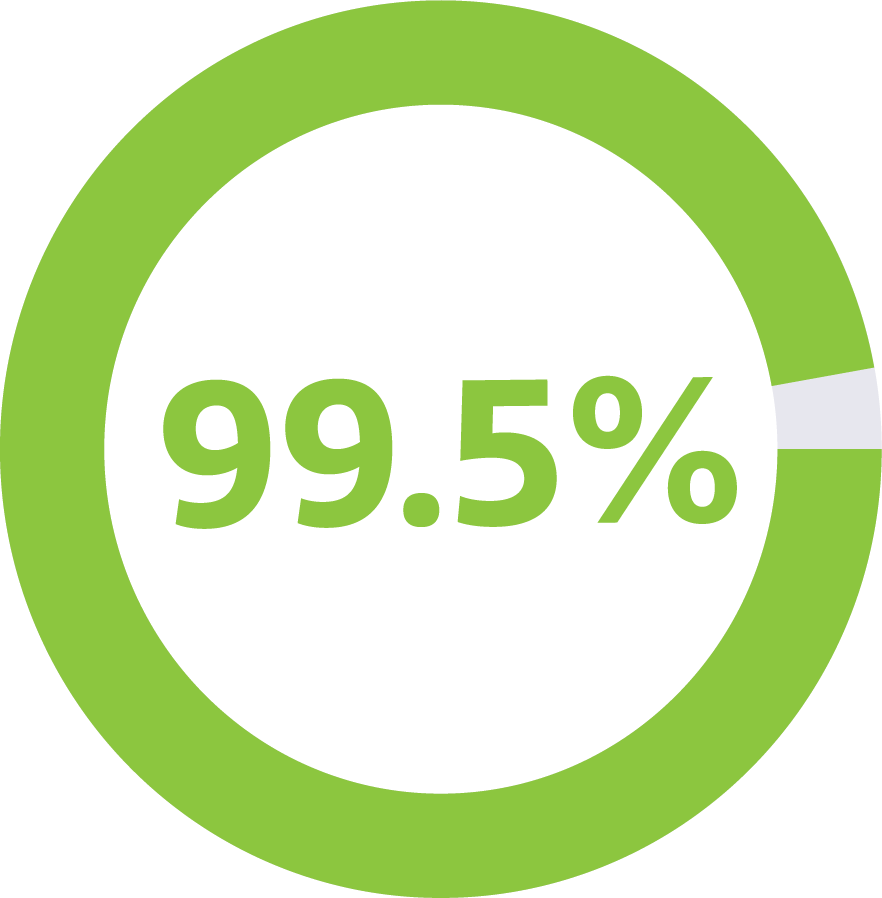
specificity
This means that the Galleri test reported a “Cancer Signal Not Detected” result in approximately 99 individuals out of 100 who do not actually have cancer.1
false positive rate
This means that the Galleri test reported a “Cancer Signal Detected” result 1 in every 200 tests run in individuals who did NOT actually have cancer.1
Cancer Signal Origin Accuracy
This means when a cancer signal was detected in an individual who actually had cancer, the first Cancer Signal Origin was correctly predicted in 89 of 100 times.1
*Sensitivity value is for all cancers and participants of all ages included in the CCGA3 subset.
Breakthrough Test Performance
sensitivity*
This means that the Galleri test reported a “Cancer Signal Detected” result in approximately 52 individuals out of 100 who actually had cancer.1

sensitivity*
This means that the Galleri test reported a “Cancer Signal Detected” result in approximately 52 individuals out of 100 who actually had cancer.1
specificity
This means that the Galleri test reported a “Cancer Signal Not Detected” result in approximately 99 individuals out of 100 who do not actually have cancer.1
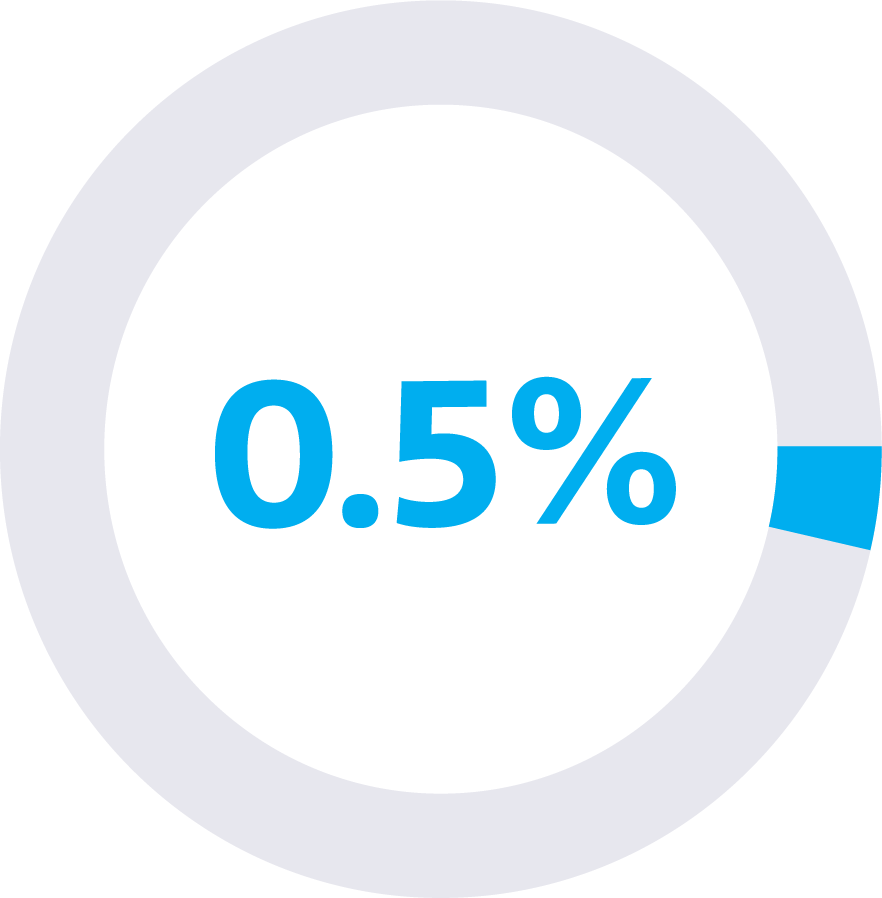
false positive rate
This means that the Galleri test reported a “Cancer Signal Detected” result 1 in every 200 tests run in individuals who did NOT actually have cancer.1
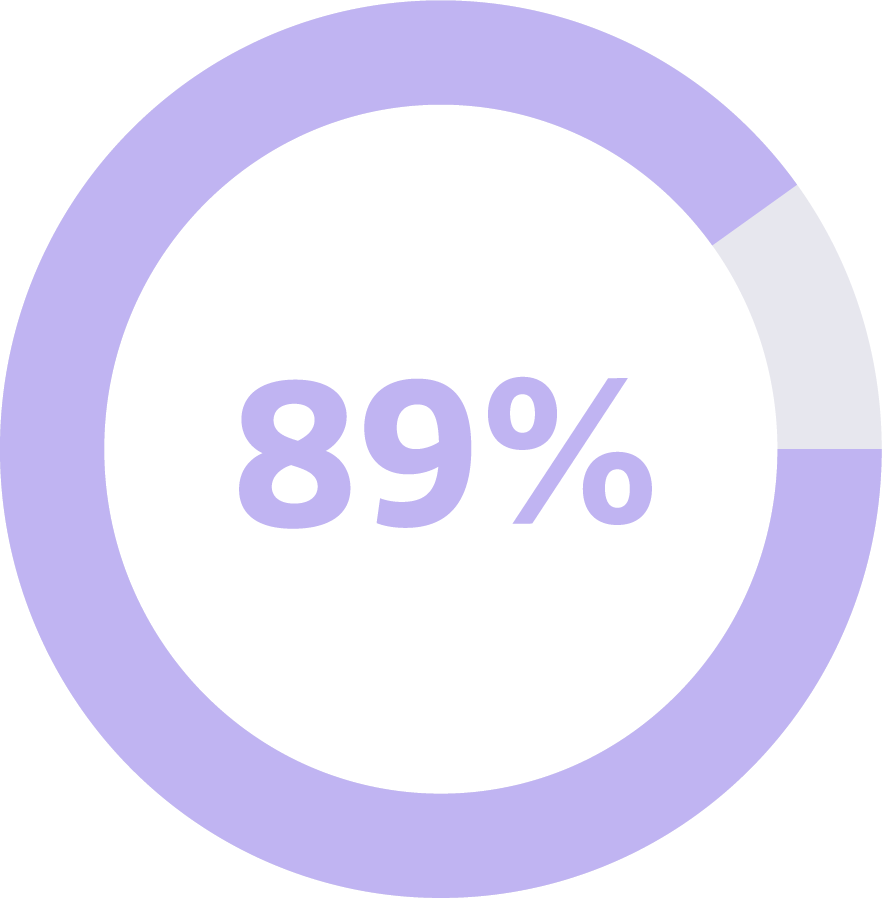
Cancer Signal Origin Accuracy
This means when a cancer signal was detected in an individual who actually had cancer, the first Cancer Signal Origin was correctly predicted in 89 of 100 times.1
*Sensitivity value is for all cancers and participants of all ages included in the CCGA3 subset.
Request the Galleri test
The Galleri multi-cancer early detection test detects a cancer signal across more than 50 types of cancer.1,2
It’s now available to eligible Illumina employees and their dependents.
Request the Galleri test
The Galleri multi-cancer early detection test detects a cancer signal across more than 50 types of cancer.1,2 It’s now available to eligible Illumina employees and their dependents.
Get the testFrequently Asked Questions
- About the Program
- Eligibility
- The Galleri Test
- Galleri Test Results
- Blood Draw & Sample Collection
- Cost & Payment
- Genetic Counseling
- Privacy
Who is Genome Medical?
Illumina has partnered with Genome Medical to offer our employees and their dependents access to genomic education and experts through chat, phone or email. Genome Medical provides educational and health-related information that progresses the understanding of genomics and helps employees get answers on a variety of questions. General genetic questions or more detailed personalized inquiries can be answered by the Genome Medical clinical team.
Why did Illumina launch the first employer-sponsored workplace genomics program?
The first Illumina employee sequencing program launched in 2014 and we have learned a lot since then. We have seen the marketplace for sequencing tests and screenings explode, while at the same time, employees expressed the need for education as a first step in their genomics journey. We have also witnessed the power of genetic counseling and doctor-to-doctor consultations to advance patient understanding and care. Illumina is excited to have partnered with Genome Medical to launch our workplace genomics program.
Am I eligible for the Galleri test?
BETWEEN THE AGES OF 22-49?
Certain risk factors may put you at an elevated risk for developing cancer. The Galleri test may be right for you if you meet one or more of the following criteria:
- You have a history of cancer (excluding basal or squamous cell carcinomas of the skin) and are more than three years out of treatment. A consultation with a genetic counselor is required if you have had a personal history of cancer and are more than three years out of treatment.
- You have a first degree relative (parent, child and/or full sibling) who has had cancer
- You are a smoker or have quit smoking in the last 10 years
- You had previous genetic testing and were found to have a gene mutation confirming an increased cancer risk due to a hereditary cancer syndrome (e.g., BRCA1/2, Lynch syndrome, CHEK2). Please note that a clinical report from an accredited laboratory that performs clinical grade genetic testing is required; this excludes tests from 23andMe, Ancestry.com and other direct to consumer genetic testing companies. A consultation with a genetic counselor is required if you have had a previous gene mutation in order to confirm the finding.
50 OR OLDER?
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those aged 50 or older. The risk of cancer increases as we age, so individuals 50 or older are eligible for the Galleri Test.
CURRENTLY, THE FOLLOWING INDIVIDUALS ARE NOT ELIGIBLE FOR THE GALLERI TEST:
- You have been diagnosed or treated for cancer within the past three years
- This excludes basal or squamous cell carcinomas of the skin
- This also excludes hormonal therapy (e.g., Tamoxifen, Arimidex, Aromasin) for breast cancer; you need to be 3 years past surgery, chemotherapy and/or radiotherapy but can still be taking hormonal therapy
- You are 21 years old or younger
- You are currently pregnant
- You live outside of the United States; Genome Medical is not able to order testing for people who reside outside the US
Can I request the test as a benefit through my personal healthcare provider?
In order to receive the test and genetic counseling at no cost, you must request the test through the Illumina and Genome Medical employer program.
Is the test available outside of the United States?
The Galleri test is not yet commercially available outside of the United States.
I do not meet clinical eligibility for the Galleri test; can I still request the test?
You can schedule a genetic counseling consultation to review your personal and family history to confirm eligibility and if criteria is met, Genome Medical will order the Galleri test for you. If you do not meet criteria, your genetic counselor can review alternative cancer screening options or genetic testing. Schedule a 1:1 consultation with a Genome Medical genetic counselor by visiting https://www.genomemedical.com/programs/illumina/.
Are other family members, such as parents and siblings, eligible for the program?
The Galleri test is only available for no cost for employees and their dependents (spouses/domestic partners and children) that meet clinical eligibility requirements. All other family members can learn more and request the test by visiting www.www.genomemedical.com/galleri. The cost of the test and provider services will be paid for by the family member(s).
I'm currently pregnant, can I request the test?
The Galleri test is not available to people that are currently pregnant. Pregnant people who meet the clinical eligibility criteria may take the test once the pregnancy is complete.
Can I request the Galleri test each year?
The Galleri test is offered annually, and eligibility is based on a rolling calendar year from when your test results were completed and reported to you. If you received Galleri test results in the past, Genome Medical will notify you once you’re eligible to request the test again.
What is the Galleri test?
The Galleri test is a multi-cancer early detection test that detects more than 50 types of cancer, many of which are not commonly screened for today, through a simple blood draw. The Galleri test does not diagnose cancer and not all cancers may be detected by the test.
Which cancers does the Galleri test detect?
In a large-scale clinical evaluation sub-study of more than 5,000 people, the Galleri test detected more than 50 cancer types across all stages. The test identified ~17% stage I cancer, 40% with stage II cancer, 77% stage III cancer and 90% of stage IV cancers.
Why is the Galleri test recommended to be used along with other cancer screening tests?
The Galleri test screens for more than 50 cancers; however, not all cancers may be detected by the test. Recommended routine cancer screening tests, such as a colonoscopy or mammogram are commonly used and have been shown to reduce cancer deaths. Galleri is intended to be used in addition to, not to replace, these tests and can help maximize the benefits of early cancer detection. Missing recommended screening or ignoring symptoms could lead to a delayed diagnosis of cancer. Ask Genome Medical about which cancer screening tests are right for you.
How quickly will I receive my results?
Results should be available to your ordering provider within 10 business days (Mon — Fri, excluding holidays) from the time your sample was received at the GRAIL laboratory. Your sample will arrive at the laboratory 1-2 business days after your blood draw.
At that time, Genome Medical will contact you via email or phone to schedule an optional genetic counseling session to review your test results and discuss the next steps. Once you receive your results, it’s important to share your results and clinical action plan (which will be provided by Genome Medical) with your primary care provider.
What does it mean if I receive a “Cancer Signal Not Detected” result?
This means that no cancer signal was found. Not all cancers can be detected by the Galleri test. You should continue with all routine screening tests that your healthcare provider recommends. Missing routine cancer screenings or ignoring symptoms could lead to a delayed diagnosis of cancer.
What does it mean if I receive a “Cancer Signal Detected” result?
The Galleri test does not diagnose cancer but indicates a suspicion that you may have cancer. The Galleri test can point to where in the body the cancer may be coming from, for example the lung or liver, to help your healthcare provider guide your next steps. Illumina has partnered with Included Health to connect you with expert healthcare providers, in addition to your primary care provider, who can order additional tests to determine if cancer is present.
Why are additional tests needed when the Galleri test detects a cancer signal?
Additional tests are required to confirm whether you have cancer. The tests ordered by a healthcare provider are needed to confirm if cancer is present which may include a combination of blood work or imaging studies. Genome Medical will provide recommendations for which tests are needed.
Will the additional tests be covered by insurance?
Insurance coverage for bloodwork and imaging studies is generally covered by insurers when there is an indication or reason to order them. Since the Galleri test is a new test, it is possible that your insurance may not cover some of the additional tests. Illumina, GRAIL and Genome Medical can provide resources that may help your provider show medical necessity for coverage for additional tests, but coverage cannot be guaranteed.
For employees with insurance coverage through the Blue Shield plan, if any advanced imaging (MRI, CT, PET/CT scan) is recommended, please contact Collective Health at 1-833-743-3223 and make them aware of the imaging test(s) that is being recommended. Collective Health will contact Blue Shield to preemptively approve the imaging test(s) so that Blue Shield does not deny the claim once it is submitted.
Why should I take the Galleri test in the future if a cancer signal is not detected?
The test does not measure your genetic risk of developing cancer in the future and is instead looking for the presence of a cancer signal at the time your blood is drawn. You may develop cancer in the future which is why the Galleri test may be ordered more than once. Your healthcare provider at Genome Medical is the best person to determine when to take the Galleri test again as a part of your routine screening based on any underlying risk factors or previous test results.
Can you share my Galleri test result with one of my healthcare providers?
Yes. Genome Medical can share your Galleri test results with your healthcare providers.
What is the test performance of the Galleri test?
In a large-scale clinical study, the Galleri test had 51.5% sensitivity for all cancers and 76.3% sensitivity in cancers that cause two-thirds of cancer deaths in the US (anus, bladder, colon/rectum, esophagus, head and neck, liver/bile duct, lung, lymphoma, ovary, pancreas, plasma cell neoplasm, and stomach).3, 5 Sensitivity measures how often the test correctly identifies the cancer signal when cancer is present (also known as the true positive rate).
The Galleri test had a specificity of 99.5% or false-positive rate of 0.5%, meaning in approximately 200 people tested, only 1 person received a false positive result (a false positive is a test result of “Cancer Signal Detected” when cancer is not present).3
How accurate is the test in predicting the location of a cancer?
When the Galleri test detects a cancer signal, it also predicted where the cancer was located with high accuracy and was correct 89% of the time in a clinical validation sub-study.3
Can I discuss my results with a genetic counselor?
Yes. You have the option to speak with a Genome Medical board-certified genetic counselor, at no cost to you, before and after the test is ordered.
How do I complete my blood draw?
Once your test has been authorized by your Genome Medical physician, a Galleri collection kit will be mailed directly to you with directions on how to locate a laboratory to have your blood drawn. You will need to bring the following with you:
- Your Galleri collection kit
Will be shipped directly to you - Your completed and printed Test Requisition Form
GRAIL will email this to you after your test has been authorized by Genome Medical. Must be printed and brought to your blood draw appointment.
Where do I go for my blood draw?
To locate a laboratory near you, visit www.galleri.com/locate-lab.
How do I return the Galleri collection kit after my blood draw?
The trained practitioner who draws your blood will pack and ship your sample back to the GRAIL laboratory for processing.
Do I need to fast prior to the blood draw?
No preparation or fasting is required for the Galleri test.
How much blood is needed for the Galleri test?
Approximately 1.5 tablespoons (or about 20 mL) of blood in two tubes typically from a vein in your arm.
Is there a cost for using services through Genome Medical?
There is no cost for employees and their dependents (spouse/domestic partner and children) to access education or genetic counseling services through Genome Medical. However, employees and their dependents (spouse/domestic partner and children) will be responsible for the cost of any genetic tests with the exception of the TruGenome™ UDD Test (the test used for RUGD cases), Foundation Medicine cancer test or the Galleri multi-cancer early detection test – clinical eligibility requirements must be met to access these tests at no cost. When medically appropriate, genetic testing will be billed to your health insurance.
Is there a cost for the Galleri test?
All regular US-based Illumina employees and their dependents (spouses/domestic partners and children) in the United States who meet clinical eligibility may access the test at no cost. Clinical eligibility is currently age 50 years or older or those who are between the ages of 18-49 who have an elevated risk of developing cancer. Eligible employees and their dependents (spouse/domestic partner and children) between the ages of 18-49 may be eligible to receive the test at no cost if any of the elevated risk criteria below are met. If you meet these criteria and you’re interested in the Galleri test, a genetic counseling consultation with one of Genome Medical’s genetic counselors is required. During this session, your personal and family history will be reviewed to confirm eligibility and if criteria are met, Genome Medical will order the Galleri test for you.
Identified Gene Mutation: you had previous genetic testing and were found to have a gene mutation confirming an increased cancer risk due to a hereditary cancer syndrome (e.g., BRCA1/2, Lynch syndrome, CHEK2). Please note that a clinical report from an accredited laboratory that performs clinical grade genetic testing is required; this excludes tests from 23andMe, Ancestry.com and other direct to consumer genetic testing companies.
Prior Cancer: you previously had cancer and completed treatment; excludes basal or squamous cell carcinoma of the skin
Direct Biological Family History of Cancer: you have a biological parent, child, and/or a full brother or sister who has had cancer and falls within one of the criteria below:
- the relative was diagnosed under the age of 50
- the relative has had more than one cancer excluding a basal or squamous cell carcinoma of the skin and/or the second cancer is not a recurrence or metastasis of the first cancer
- or the relative passed away from cancer
The Galleri test is currently not available outside of the US.
Is a pre-test consultation required?
If you’re over the age of 50 or meet clinical criteria of being at an elevated risk of developing cancer a genetic counseling consultation is NOT required.
However, if you have had previous genetic testing and were found to have a gene mutation confirming an increased cancer risk due to a hereditary cancer syndrome (e.g., BRCA1/2, Lynch syndrome, CHEK2) a consultation with a genetic counselor is required. Please note that a clinical report from an accredited laboratory that performs clinical grade genetic testing is required; this excludes tests from 23andMe, Ancestry.com and other direct to consumer genetic testing companies.
Can I discuss my results with a genetic counselor?
Yes. You have the option to speak with a Genome Medical board-certified genetic counselor, at no cost to you, before and after the test is ordered. Our GCs have been educated on the Galleri test and can provide guidance on the test and results.
Is there a cost for me to discuss my results with a genetic counselor?
There is no cost to speak with a Genome Medical board-certified genetic counselor through this program and employee benefit. Our GCs have been educated on the Galleri test and can provide guidance on the test and results.
What should I expect during a post-test counseling session?
During your post-test consultation you will meet with a Genome Medical board-certified genetic counselor via video or phone. Your genetic counselor will:
- Review your Galleri test results.
- Provide a clear understanding of what your results mean for you. The Galleri test does not measure your genetic risk of developing cancer in the future. The Galleri test looks for DNA changes in the blood that may be associated with cancer at the time of your blood draw.
- Develop a clinical action plan for your ongoing care that you can share with your physician.
- Learn about recommended next steps.
How will Illumina protect confidentiality as well as my health information and the health of my family member(s), including the Galleri test information?
Illumina takes patient privacy seriously. Illumina, Genome Medical and the GRAIL Laboratory will maintain the health information in compliance with all applicable medical privacy laws, including HIPAA, GINA, etc. Genome Medical will not share any health information with Illumina. Use of the Genomics Resource Center is confidential.
Frequently Asked Questions
- About the Program
- Eligibility
- The Galleri Test
- Privacy
- Galleri Test Results
- Blood Draw & Sample Collection
- Cost & Payment
- Genetic Counseling
Who is Genome Medical?
Illumina has partnered with Genome Medical to offer our employees and their dependents access to genomic education and experts through chat, phone or email. Genome Medical provides educational and health-related information that progresses the understanding of genomics and helps employees get answers on a variety of questions. General genetic questions or more detailed personalized inquiries can be answered by the Genome Medical clinical team.
Why did Illumina launch the first employer-sponsored workplace genomics program?
The first Illumina employee sequencing program launched in 2014 and we have learned a lot since then. We have seen the marketplace for sequencing tests and screenings explode, while at the same time, employees expressed the need for education as a first step in their genomics journey. We have also witnessed the power of genetic counseling and doctor-to-doctor consultations to advance patient understanding and care. Illumina is excited to have partnered with Genome Medical to launch our workplace genomics program.
Am I eligible for the Galleri test?
BETWEEN THE AGES OF 22-49?
Certain risk factors may put you at an elevated risk for developing cancer. The Galleri test may be right for you if you meet one or more of the following criteria:
- You have a history of cancer (excluding basal or squamous cell carcinomas of the skin) and are more than three years out of treatment. A consultation with a genetic counselor is required if you have had a personal history of cancer and are more than three years out of treatment.
- You have a first degree relative (parent, child and/or full sibling) who has had cancer
- You are a smoker or have quit smoking in the last 10 years
- You had previous genetic testing and were found to have a gene mutation confirming an increased cancer risk due to a hereditary cancer syndrome (e.g., BRCA1/2, Lynch syndrome, CHEK2). Please note that a clinical report from an accredited laboratory that performs clinical grade genetic testing is required; this excludes tests from 23andMe, Ancestry.com and other direct to consumer genetic testing companies. A consultation with a genetic counselor is required if you have had a previous gene mutation in order to confirm the finding.
50 OR OLDER?
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those aged 50 or older. The risk of cancer increases as we age, so individuals 50 or older are eligible for the Galleri Test.
CURRENTLY, THE FOLLOWING INDIVIDUALS ARE NOT ELIGIBLE FOR THE GALLERI TEST:
- You have been diagnosed or treated for cancer within the past three years
- This excludes basal or squamous cell carcinomas of the skin
- This also excludes hormonal therapy (e.g., Tamoxifen, Arimidex, Aromasin) for breast cancer; you need to be 3 years past surgery, chemotherapy and/or radiotherapy but can still be taking hormonal therapy
- You are 21 years old or younger
- You are currently pregnant
- You live outside of the United States; Genome Medical is not able to order testing for people who reside outside the US
Can I request the test as a benefit through my personal healthcare provider?
In order to receive the test and genetic counseling at no cost, you must request the test through the Illumina and Genome Medical employer program.
Is the test available outside of the United States?
The Galleri test is not yet commercially available outside of the United States.
I do not meet clinical eligibility for the Galleri test; can I still request the test?
You can schedule a genetic counseling consultation to review your personal and family history to confirm eligibility and if criteria is met, Genome Medical will order the Galleri test for you. If you do not meet criteria, your genetic counselor can review alternative cancer screening options or genetic testing. Schedule a 1:1 consultation with a Genome Medical genetic counselor by visiting https://www.genomemedical.com/programs/illumina/.
Are other family members, such as parents and siblings, eligible for the program?
The Galleri test is only available for no cost for employees and their dependents (spouses/domestic partners and children) that meet clinical eligibility requirements. All other family members can learn more and request the test by visiting www.genomemedical.com/galleri. The cost of the test and provider services will be paid for by the family member(s).
I'm currently pregnant, can I request the test?
The Galleri test is not available to people that are currently pregnant. Pregnant people who meet the clinical eligibility criteria may take the test once the pregnancy is complete.
Can I request the Galleri test each year?
The Galleri test is offered annually, and eligibility is based on a rolling calendar year from when your test results were completed and reported to you. If you received Galleri test results in the past, Genome Medical will notify you once you’re eligible to request the test again.
What is the Galleri test?
The Galleri test is a multi-cancer early detection test that detects more than 50 types of cancer, many of which are not commonly screened for today, through a simple blood draw. The Galleri test does not diagnose cancer and not all cancers may be detected by the test.
Which cancers does the Galleri test detect?
In a large-scale clinical evaluation sub-study of more than 5,000 people, the Galleri test detected more than 50 cancer types across all stages. The test identified ~17% stage I cancer, 40% with stage II cancer, 77% stage III cancer and 90% of stage IV cancers.
Why is the Galleri test recommended to be used along with other cancer screening tests?
The Galleri test screens for more than 50 cancers; however, not all cancers may be detected by the test. Recommended routine cancer screening tests, such as a colonoscopy or mammogram are commonly used and have been shown to reduce cancer deaths. Galleri is intended to be used in addition to, not to replace, these tests and can help maximize the benefits of early cancer detection. Missing recommended screening or ignoring symptoms could lead to a delayed diagnosis of cancer. Ask Genome Medical about which cancer screening tests are right for you.
How will Illumina protect confidentiality as well as my health information and the health of my family member(s), including the Galleri test information?
Illumina takes patient privacy seriously. Illumina, Genome Medical and the GRAIL Laboratory will maintain the health information in compliance with all applicable medical privacy laws, including HIPAA, GINA, etc. Genome Medical will not share any health information with Illumina. Use of the Genomics Resource Center is confidential.
How quickly will I receive my results?
Results should be available to your ordering provider within 10 business days (Mon — Fri, excluding holidays) from the time your sample was received at the GRAIL laboratory. Your sample will arrive at the laboratory 1-2 business days after your blood draw.
At that time, Genome Medical will contact you via email or phone to schedule an optional genetic counseling session to review your test results and discuss the next steps. Once you receive your results, it’s important to share your results and clinical action plan (which will be provided by Genome Medical) with your primary care provider.
What does it mean if I receive a “Cancer Signal Not Detected” result?
This means that no cancer signal was found. Not all cancers can be detected by the Galleri test. You should continue with all routine screening tests that your healthcare provider recommends. Missing routine cancer screenings or ignoring symptoms could lead to a delayed diagnosis of cancer.
What does it mean if I receive a “Cancer Signal Detected” result?
The Galleri test does not diagnose cancer but indicates a suspicion that you may have cancer. The Galleri test can point to where in the body the cancer may be coming from, for example the lung or liver, to help your healthcare provider guide your next steps. Illumina has partnered with Included Health to connect you with expert healthcare providers, in addition to your primary care provider, who can order additional tests to determine if cancer is present.
Why are additional tests needed when the Galleri test detects a cancer signal?
Additional tests are required to confirm whether you have cancer. The tests ordered by a healthcare provider are needed to confirm if cancer is present which may include a combination of blood work or imaging studies. Genome Medical will provide recommendations for which tests are needed.
Will the additional tests be covered by insurance?
Insurance coverage for bloodwork and imaging studies is generally covered by insurers when there is an indication or reason to order them. Since the Galleri test is a new test, it is possible that your insurance may not cover some of the additional tests. Illumina, GRAIL and Genome Medical can provide resources that may help your provider show medical necessity for coverage for additional tests, but coverage cannot be guaranteed.
For employees with insurance coverage through the Blue Shield plan, if any advanced imaging (MRI, CT, PET/CT scan) is recommended, please contact Collective Health at 1-833-743-3223 and make them aware of the imaging test(s) that is being recommended. Collective Health will contact Blue Shield to preemptively approve the imaging test(s) so that Blue Shield does not deny the claim once it is submitted.
Why should I take the Galleri test in the future if a cancer signal is not detected?
The test does not measure your genetic risk of developing cancer in the future and is instead looking for the presence of a cancer signal at the time your blood is drawn. You may develop cancer in the future which is why the Galleri test may be ordered more than once. Your healthcare provider at Genome Medical is the best person to determine when to take the Galleri test again as a part of your routine screening based on any underlying risk factors or previous test results.
Can you share my Galleri test result with one of my healthcare providers?
Yes. Genome Medical can share your Galleri test results with your healthcare providers.
What is the test performance of the Galleri test?
In a large-scale clinical study, the Galleri test had 51.5% sensitivity for all cancers and 76.3% sensitivity in cancers that cause two-thirds of cancer deaths in the US (anus, bladder, colon/rectum, esophagus, head and neck, liver/bile duct, lung, lymphoma, ovary, pancreas, plasma cell neoplasm, and stomach).3, 5 Sensitivity measures how often the test correctly identifies the cancer signal when cancer is present (also known as the true positive rate).
The Galleri test had a specificity of 99.5% or false-positive rate of 0.5%, meaning in approximately 200 people tested, only 1 person received a false positive result (a false positive is a test result of “Cancer Signal Detected” when cancer is not present).3
How accurate is the test in predicting the location of a cancer?
When the Galleri test detects a cancer signal, it also predicted where the cancer was located with high accuracy and was correct 89% of the time in a clinical validation sub-study.3
Can I discuss my results with a genetic counselor?
Yes. You have the option to speak with a Genome Medical board-certified genetic counselor, at no cost to you, before and after the test is ordered.
How do I complete my blood draw?
Once your test has been authorized by your Genome Medical physician, a Galleri collection kit will be mailed directly to you with directions on how to locate a laboratory to have your blood drawn. You will need to bring the following with you:
- Your Galleri collection kit
Will be shipped directly to you - Your completed and printed Test Requisition Form
GRAIL will email this to you after your test has been authorized by Genome Medical. Must be printed and brought to your blood draw appointment.
Where do I go for my blood draw?
To locate a laboratory near you, visit www.galleri.com/locate-lab.
How do I return the Galleri collection kit after my blood draw?
The trained practitioner who draws your blood will pack and ship your sample back to the GRAIL laboratory for processing.
Do I need to fast prior to the blood draw?
No preparation or fasting is required for the Galleri test.
How much blood is needed for the Galleri test?
Approximately 1.5 tablespoons (or about 20 mL) of blood in two tubes typically from a vein in your arm.
Is there a cost for using services through Genome Medical?
There is no cost for employees and their dependents (spouse/domestic partner and children) to access education or genetic counseling services through Genome Medical. However, employees and their dependents (spouse/domestic partner and children) will be responsible for the cost of any genetic tests with the exception of the TruGenome™ UDD Test (the test used for RUGD cases), Foundation Medicine cancer test or the Galleri multi-cancer early detection test – clinical eligibility requirements must be met to access these tests at no cost. When medically appropriate, genetic testing will be billed to your health insurance.
Is there a cost for the Galleri test?
All regular US-based Illumina employees and their dependents (spouses/domestic partners and children) in the United States who meet clinical eligibility may access the test at no cost. Clinical eligibility is currently age 50 years or older or those who are between the ages of 18-49 who have an elevated risk of developing cancer. Eligible employees and their dependents (spouse/domestic partner and children) between the ages of 18-49 may be eligible to receive the test at no cost if any of the elevated risk criteria below are met. If you meet these criteria and you’re interested in the Galleri test, a genetic counseling consultation with one of Genome Medical’s genetic counselors is required. During this session, your personal and family history will be reviewed to confirm eligibility and if criteria are met, Genome Medical will order the Galleri test for you.
Identified Gene Mutation: you had previous genetic testing and were found to have a gene mutation confirming an increased cancer risk due to a hereditary cancer syndrome (e.g., BRCA1/2, Lynch syndrome, CHEK2). Please note that a clinical report from an accredited laboratory that performs clinical grade genetic testing is required; this excludes tests from 23andMe, Ancestry.com and other direct to consumer genetic testing companies.
Prior Cancer: you previously had cancer and completed treatment; excludes basal or squamous cell carcinoma of the skin
Direct Biological Family History of Cancer: you have a biological parent, child, and/or a full brother or sister who has had cancer and falls within one of the criteria below:
- the relative was diagnosed under the age of 50
- the relative has had more than one cancer excluding a basal or squamous cell carcinoma of the skin and/or the second cancer is not a recurrence or metastasis of the first cancer
- or the relative passed away from cancer
The Galleri test is currently not available outside of the US.
Is a pre-test consultation required?
If you’re over the age of 50 or meet clinical criteria of being at an elevated risk of developing cancer a genetic counseling consultation is NOT required.
However, if you have had previous genetic testing and were found to have a gene mutation confirming an increased cancer risk due to a hereditary cancer syndrome (e.g., BRCA1/2, Lynch syndrome, CHEK2) a consultation with a genetic counselor is required. Please note that a clinical report from an accredited laboratory that performs clinical grade genetic testing is required; this excludes tests from 23andMe, Ancestry.com and other direct to consumer genetic testing companies.
Can I discuss my results with a genetic counselor?
Yes. You have the option to speak with a Genome Medical board-certified genetic counselor, at no cost to you, before and after the test is ordered. Our GCs have been educated on the Galleri test and can provide guidance on the test and results.
Is there a cost for me to discuss my results with a genetic counselor?
There is no cost to speak with a Genome Medical board-certified genetic counselor through this program and employee benefit. Our GCs have been educated on the Galleri test and can provide guidance on the test and results.
What should I expect during a post-test counseling session?
During your post-test consultation you will meet with a Genome Medical board-certified genetic counselor via video or phone. Your genetic counselor will:
- Review your Galleri test results.
- Provide a clear understanding of what your results mean for you. The Galleri test does not measure your genetic risk of developing cancer in the future. The Galleri test looks for DNA changes in the blood that may be associated with cancer at the time of your blood draw.
- Develop a clinical action plan for your ongoing care that you can share with your physician.
- Learn about recommended next steps.
Important Safety Information
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those aged 50 or older. The Galleri test does not detect all cancers and should be used in addition to routine cancer screening tests recommended by a healthcare provider. Galleri is intended to detect cancer signals and predict where in the body the cancer signal is located. Use of Galleri is not recommended in individuals who are pregnant, 21 years old or younger, or undergoing active cancer treatment.
Results should be interpreted by a healthcare provider in the context of medical history, clinical signs and symptoms. A test result of “No Cancer Signal Detected” does not rule out cancer. A test result of “Cancer Signal Detected” requires confirmatory diagnostic evaluation by medically established procedures (e.g. imaging) to confirm cancer.
If cancer is not confirmed with further testing, it could mean that cancer is not present or testing was insufficient to detect cancer, including due to the cancer being located in a different part of the body. False-positive (a cancer signal detected when cancer is not present) and false-negative (a cancer signal not detected when cancer is present) test results do occur. Rx only.
Laboratory/Test Information
GRAIL’s clinical laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and accredited by the College of American Pathologists (CAP). The Galleri test was developed, and its performance characteristics were determined by GRAIL. The Galleri test has not been cleared or approved by the Food and Drug Administration. GRAIL’s clinical laboratory is regulated under CLIA to perform high-complexity testing. The Galleri test is intended for clinical purposes.
References and Notes
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021;32(9):1167-1177.
- Amin MB, et al. (Eds). American Joint Committee of Cancer (AJCC) Cancer Staging Manual (8th edition).
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence — SEER 18 Regs Research Data. Nov 2018 Sub. Includes persons aged 50 – 79 diagnosed 2006 – 2015. “Early/ Localized” includes invasive localized tumors that have not spread beyond organ of origin. “Late/ Metastasized” includes invasive cancers that have metastasized beyond the organ of origin to other parts of the body.
- Modeled detection extrapolated to 2020 US population ages 50 – 79. Screening includes methods with United States Preventive Services Task Force (USPSTF) A, B, or C rating (breast, colon, cervical, prostate, and lung), and assumes screening is available for all prostate, breast, cervical, and colorectal cancer cases and 33% of lung cancer cases (based on estimated proportion of lung cancers that occur in screen-eligible individuals older than 40 years).
- Data on file from Surveillance, Epidemiology, and End Results (SEER) 18 Regs Research Data, Nov 2017 Submission. Includes persons aged 50 – 79. Estimated deaths per year in 2020 from American Cancer Society Cancer Facts and Figures 2020. Available at: www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf
- Assumes screening is available for all prostate, breast, cervical, and colorectal cancer cases and 43% of lung cancer cases (based on estimated proportion of lung cancers that occur in screen-eligible individuals older than 40 years) Source: Estimated deaths per year in 2021 from American Cancer Society Cancer Facts and Figures 2021. Available at: http://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf. Data on file GA-2021-0065
- Noone AM, Howlader N, Krapcho M, et al. (eds). SEER Cancer Statistics Review, 1975 – 2015, National Cancer Institute, Bethesda, MD, http://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER website April 2018.
- Screening includes methods with United States Preventative Services Task Force (USPSTF) A, B, or C rating (breast, colon, cervical, prostate, and lung).
Important Safety Information
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those aged 50 or older. The Galleri test does not detect all cancers and should be used in addition to routine cancer screening tests recommended by a healthcare provider. Galleri is intended to detect cancer signals and predict where in the body the cancer signal is located. Use of Galleri is not recommended in individuals who are pregnant, 21 years old or younger, or undergoing active cancer treatment.
Results should be interpreted by a healthcare provider in the context of medical history, clinical signs and symptoms. A test result of “No Cancer Signal Detected” does not rule out cancer. A test result of “Cancer Signal Detected” requires confirmatory diagnostic evaluation by medically established procedures (e.g. imaging) to confirm cancer.
If cancer is not confirmed with further testing, it could mean that cancer is not present or testing was insufficient to detect cancer, including due to the cancer being located in a different part of the body. False-positive (a cancer signal detected when cancer is not present) and false-negative (a cancer signal not detected when cancer is present) test results do occur. Rx only.
Laboratory/Test Information
GRAIL’s clinical laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and accredited by the College of American Pathologists (CAP). The Galleri test was developed, and its performance characteristics were determined by GRAIL. The Galleri test has not been cleared or approved by the Food and Drug Administration. GRAIL’s clinical laboratory is regulated under CLIA to perform high-complexity testing. The Galleri test is intended for clinical purposes.
References and Notes
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021;32(9):1167-1177.
- Amin MB, et al. (Eds). American Joint Committee of Cancer (AJCC) Cancer Staging Manual (8th edition).
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence — SEER 18 Regs Research Data. Nov 2018 Sub. Includes persons aged 50 – 79 diagnosed 2006 – 2015. “Early/ Localized” includes invasive localized tumors that have not spread beyond organ of origin. “Late/ Metastasized” includes invasive cancers that have metastasized beyond the organ of origin to other parts of the body.
- Modeled detection extrapolated to 2020 US population ages 50 – 79. Screening includes methods with United States Preventive Services Task Force (USPSTF) A, B, or C rating (breast, colon, cervical, prostate, and lung), and assumes screening is available for all prostate, breast, cervical, and colorectal cancer cases and 33% of lung cancer cases (based on estimated proportion of lung cancers that occur in screen-eligible individuals older than 40 years).
- Data on file from Surveillance, Epidemiology, and End Results (SEER) 18 Regs Research Data, Nov 2017 Submission. Includes persons aged 50 – 79. Estimated deaths per year in 2020 from American Cancer Society Cancer Facts and Figures 2020. Available at: www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf
- Assumes screening is available for all prostate, breast, cervical, and colorectal cancer cases and 43% of lung cancer cases (based on estimated proportion of lung cancers that occur in screen-eligible individuals older than 40 years) Source: Estimated deaths per year in 2021 from American Cancer Society Cancer Facts and Figures 2021. Available at: http://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf. Data on file GA-2021-0065
- Noone AM, Howlader N, Krapcho M, et al. (eds). SEER Cancer Statistics Review, 1975 – 2015, National Cancer Institute, Bethesda, MD, http://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER website April 2018.
- Screening includes methods with United States Preventative Services Task Force (USPSTF) A, B, or C rating (breast, colon, cervical, prostate, and lung).