What if We Found Cancer Early Enough to Make a Difference
The Galleri® multi-cancer early detection test detects more than 50 types of cancer.1,2 It’s now available to eligible Novartis employees.
Individuals must meet the clinical eligibility criteria determined by Genome Medical to receive the test.
What if We Found Cancer Early Enough to Make a Difference
The Galleri® multi-cancer early detection test detects more than 50 types of cancer.1,2 It’s now available to eligible Novartis employees.
Individuals must meet the clinical eligibility criteria determined by Genome Medical to receive the test.
Finding Cancer Early is Important
Thinking about the possibility of having cancer can be overwhelming, but taking steps to find cancer early can help you feel more in control. Often, the earlier that cancer can be found, the higher the chance of better outcomes.3
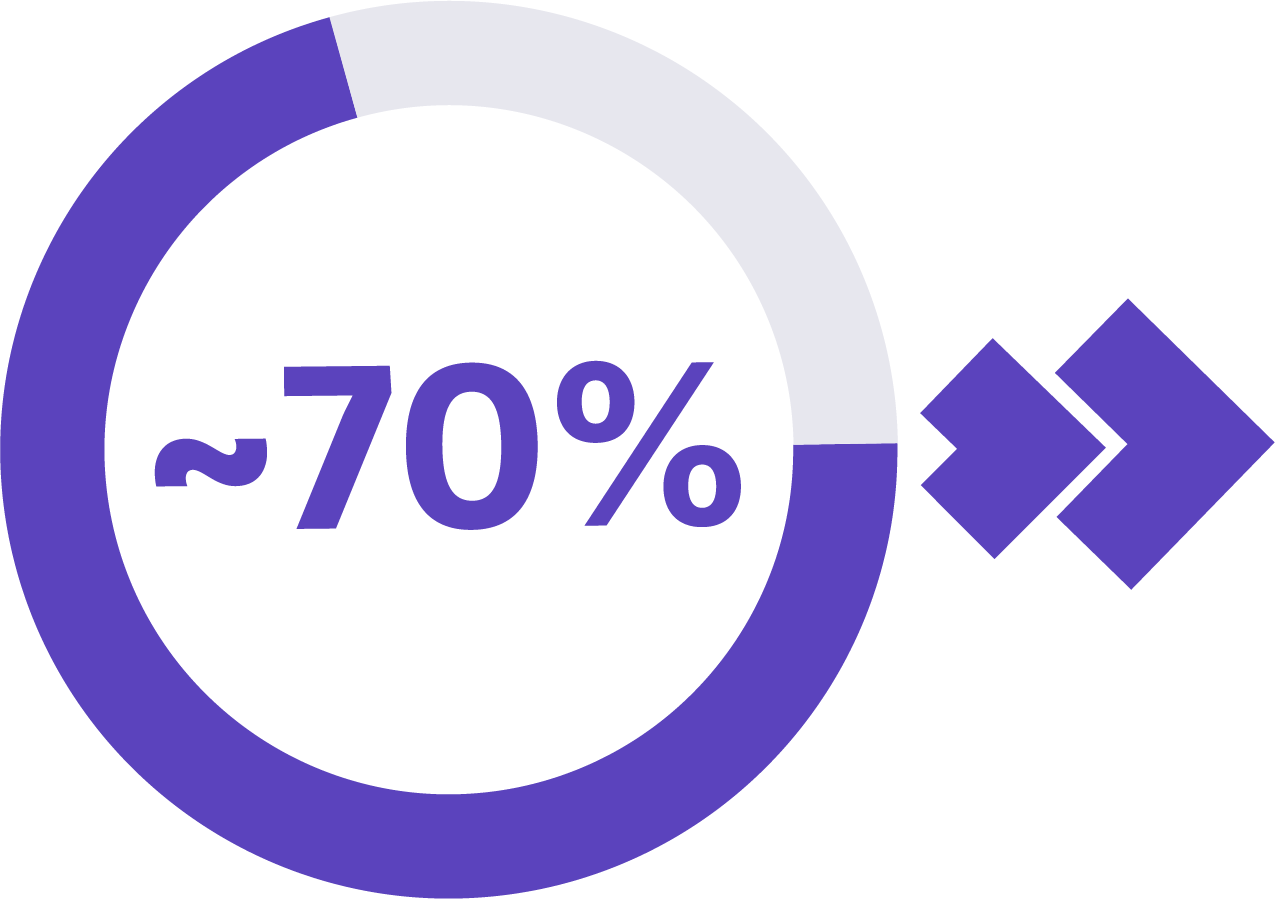
of cancer deaths in people ages 50-79 are caused by cancers not commonly screened for.4,5,6
In fact, when cancers are diagnosed early before they have had a chance to spread, the overall 5-year survival rate is 4x higher than when diagnosed in later stages.3,7
Finding Cancer Early is Important
Thinking about the possibility of having cancer can be overwhelming, but taking steps to find cancer early can help you feel more in control. Often, the earlier that cancer can be found, the higher the chance of better outcomes.3
of cancer deaths in people ages 50-79 are caused by cancers not commonly screened for.4,5,6
In fact, when cancers are diagnosed early before they have had a chance to spread, the overall 5-year survival rate is 4x higher than when diagnosed in later stages.3,7
*Video embedded from YouTube
The Galleri test, GRAIL’s multi-cancer early detection test, detects more than 50 types of cancer, many of which are not commonly screened for today. All that’s required is a simple blood draw.1,2,8
Benefit Requirements & Eligibility
This employee benefit through Novartis and Genome Medical provides eligible employees access to the Galleri multi-cancer early detection test as an annual benefit.
Genome Medical’s team of oncology experts has reviewed risk factors that increase a person’s chance to develop cancer to determine who would benefit from the Galleri test. The current criteria are not meant to represent a comprehensive list of all cancer risk factors but are a starting point to offer the Galleri test.
Between the ages of 40-49?
Certain risk factors may put you at an elevated risk for developing cancer. The Galleri test may be right for you if you meet one or more of the following criteria:
- You are a smoker or have quit smoking in the last 10 years
- You have a first degree relative (parent, child and/or full sibling) who has had cancer
- You have tested positive for HIV
- You have received an organ transplant
- You have a Body Mass Index (BMI) greater than 30
Request the Galleri test to see if you’re eligible
Genome Medical may expand the eligibility criteria as more data becomes available. If you would like to be notified when eligibility requirements change, please click here.
50 OR OLDER?
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those aged 50 or older. The risk of cancer increases as we age, so individuals 50 or older are eligible for the Galleri Test.
CURRENTLY, THE FOLLOWING INDIVIDUALS ARE NOT ELIGIBLE FOR THE GALLERI TEST
- You have been diagnosed or treated for cancer within the past three years
- This excludes basal or squamous cell carcinomas of the skin
- This also excludes hormonal therapy (e.g., Tamoxifen, Arimidex, Aromasin) for breast cancer; you need to be 3 years past surgery, chemotherapy and/or radiotherapy but can still be taking hormonal therapy
- You are 39 years of age or younger
- You are currently pregnant
- You live outside of the United States; Genome Medical is not able to order testing for people who reside outside the US
Benefit Requirements & Eligibility
This employee benefit through Novartis and Genome Medical provides eligible employees access to the Galleri multi-cancer early detection test as an annual benefit.
Genome Medical’s team of oncology experts has reviewed risk factors that increase a person’s chance to develop cancer to determine who would benefit from the Galleri test. The current criteria are not meant to represent a comprehensive list of all cancer risk factors but are a starting point to offer the Galleri test.
BETWEEN THE AGES OF 40-49?
Certain risk factors may put you at an elevated risk for developing cancer. The Galleri test may be right for you if you meet one or more of the following criteria:
- You are a smoker or have quit smoking in the last 10 years
- You have a first degree relative (parent, child and/or full sibling) who has had cancer
- You have tested positive for HIV
- You have received an organ transplant
- You have a Body Mass Index (BMI) greater than 30
Request the Galleri test to see if you’re eligible
Genome Medical may expand the eligibility criteria as more data becomes available. If you would like to be notified when eligibility requirements change, please click here.
50 OR OLDER?
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those aged 50 or older. The risk of cancer increases as we age, so individuals 50 or older are eligible for the Galleri Test.
CURRENTLY, THE FOLLOWING INDIVIDUALS ARE NOT ELIGIBLE FOR THE GALLERI TEST
- You have been diagnosed or treated for cancer within the past three years
- This excludes basal or squamous cell carcinomas of the skin
- This also excludes hormonal therapy (e.g., Tamoxifen, Arimidex, Aromasin) for breast cancer; you need to be 3 years past surgery, chemotherapy and/or radiotherapy but can still be taking hormonal therapy
- You are 39 years of age or younger
- You are currently pregnant
- You live outside of the United States; Genome Medical is not able to order testing for people who reside outside the US
The Benefits of Multi-Cancer Detection
Early cancer detection
Detects many cancers not commonly screened for today, to allow for earlier treatment.1,8
Testing with ease
Completed with a simple blood draw.
Actionable results
If a cancer signal is found, the results can point to where in the body the cancer is coming from with high accuracy.
The Galleri test is intended to be used in addition to, and not replace, other cancer screening tests your health care provider recommends. The Galleri test does not detect all cancers nor does it measure your genetic risk of developing cancer in the future.
The Benefits of Multi-Cancer Detection
Early cancer detection
Detects many cancers not commonly screened for today, to allow for earlier treatment.1,8
Testing with ease
Completed with a simple blood draw.
Actionable results
If a cancer signal is found, the results can point to where in the body the cancer is coming from with high accuracy.
The Galleri test is intended to be used in addition to, and not replace, other cancer screening tests your health care provider recommends. The Galleri test does not detect all cancers nor does it measure your genetic risk of developing cancer in the future.
Test Process
Follow the process with just three steps.
Test Process
Follow the process with just three steps.
Ordering the Galleri test
The Galleri test must be ordered by a health care provider. If you’re ready to request the test, you’ll be asked to answer a few questions and a Genome Medical physician will review your information and determine if the test is right for you.
To learn more about clinical eligibility before requesting the test, please click here.

Ordering the
Galleri test
The Galleri test must be ordered by a health care provider. If you’re ready to request the test, you’ll be asked to answer a few questions and a Genome Medical physician will review your information and determine if the test is right for you.
To learn more about clinical eligibility before requesting the test, please click here.
Get a blood draw
If your test has been authorized by your Genome Medical physician, a Galleri collection kit will be mailed directly to you with directions on how to locate a partner laboratory to have your blood drawn. You may also visit the Primary Care at Novartis Morristown location for your blood draw. Please do not open the kit. You must take the kit to the lab unopened.
Make sure you have the following in hand for your blood draw:
Your Galleri collection kit
Will be shipped directly to you.
Your completed and printed
Test Requisition Form
GRAIL will email you the Test Requisition Form within one business day once your test has been ordered by Genome Medical. You’ll need to access and print your Test Requisition Form and bring that with you to your blood draw appointment. Look out for an email with the subject line “Your Galleri Test Has Been Ordered” sent from customerservice@galleri.grail.com.
If you are a current patient who has questions about scheduling a blood draw, please find more information here or contact GRAIL Customer Service at 833-694-2553 or customerservice@grail.com.
Get a blood draw
If your test has been authorized by your Genome Medical physician, a Galleri collection kit will be mailed directly to you with directions on how to locate a partner laboratory to have your blood drawn. You may also visit the Primary Care at Novartis Morristown location for your blood draw. Please do not open the kit. You must take the kit to the lab unopened.
Make sure you have the following in hand for your blood draw:
Your Galleri collection kit
Will be shipped directly to you.
Your completed and printed Test Requisition Form
GRAIL will email you the Test Requisition Form within one business day once your test has been ordered by Genome Medical. You’ll need to access and print your Test Requisition Form and bring that with you to your blood draw appointment. Look out for an email with the subject line “Your Galleri Test Has Been Ordered” sent from customerservice@galleri.
grail.com.
If you are a current patient who has questions about scheduling a blood draw, please find more information here or contact GRAIL Customer Service at 833-694-2553 or customerservice@grail.com.
Receive your results and clinical action plan
Your results will be available approximately two weeks after your sample arrives at the GRAIL laboratory. At that time, Genome Medical will contact you via email or phone to review your test results and discuss next steps. Additionally, you’ll have the option to schedule a return of results session to further discuss your results. Once you receive your results, it’s important to share your results and clinical action plan with your primary care physician.
There are two possible results from the Galleri test:
Cancer Signal NOT Detected
This means that no cancer signal was found. However not all cancer types can be detected by the Galleri test, and the Galleri test may not detect all early stage cancers.
Cancer Signal Detected
This means that there is a suspicion of cancer. The Galleri test can point to where in the body (e.g., colon, head and neck) the cancer signal is coming from with high accuracy to help your health care provider guide next steps. Sometimes the test may indicate two locations in the body where the cancer signal may be coming from which may require separate follow-up evaluations.
Next steps:
Continue with all routine screening tests that your health care provider recommends. Missing routine cancer screenings or ignoring symptoms could lead to a delayed diagnosis of cancer. If you’re interested in repeating the Galleri test in one year, please contact Genome Medical.
Next steps:
The Galleri test does not diagnose cancer. Additional tests are needed to determine if cancer is present. Genome Medical will work with your primary care provider and Memorial Sloan Kettering Cancer Center (MSK) to guide you through the evaluation and work-up. Novartis has a partnership with MSK to provide access to cancer care and support for Novartis employees.
The test does not measure your genetic risk of developing cancer in the future. With the Galleri multi-cancer early detection test, annual screening provides the opportunity to detect more cancers early.
False-positive and false-negative test results can occur. The Galleri test has a low false-positive rate of 0.5% (detecting a cancer signal when no cancer is present).1
Receive your results and clinical action plan
Your results will be available approximately two weeks after your sample arrives at the GRAIL laboratory. At that time, Genome Medical will contact you via email or phone to review your test results and discuss next steps. Additionally, you’ll have the option to schedule a return of results session to further discuss your results. Once you receive your results, it’s important to share your results and clinical action plan with your primary care physician.
There are two possible results from the Galleri test:
Cancer Signal NOT Detected
This means that no cancer signal was found. However not all cancer types can be detected by the Galleri test, and the Galleri test may not detect all early stage cancers.
Next steps:
Continue with all routine screening tests that your health care provider recommends. Missing routine cancer screenings or ignoring symptoms could lead to a delayed diagnosis of cancer. If you’re interested in repeating the Galleri test in one year, please contact Genome Medical.
Cancer Signal Detected
This means that there is a suspicion of cancer. The Galleri test can point to where in the body (e.g., colon, head and neck) the cancer signal is coming from with high accuracy to help your health care provider guide next steps. Sometimes the test may indicate two locations in the body where the cancer signal may be coming from which may require separate follow-up evaluations.
Next steps:
The Galleri test does not diagnose cancer. Additional tests are needed to determine if cancer is present. Genome Medical will work with your primary care provider and Memorial Sloan Kettering Cancer Center (MSK) to guide you through the evaluation and work-up. Novartis has a partnership with MSK to provide access to cancer care and support for Novartis employees.
The test does not measure your genetic risk of developing cancer in the future. With the Galleri multi-cancer early detection test, annual screening provides the opportunity to detect more cancers early.
False-positive and false-negative test results can occur. The Galleri test has a low false-positive rate of 0.5% (detecting a cancer signal when no cancer is present).1
Breakthrough Test Performance
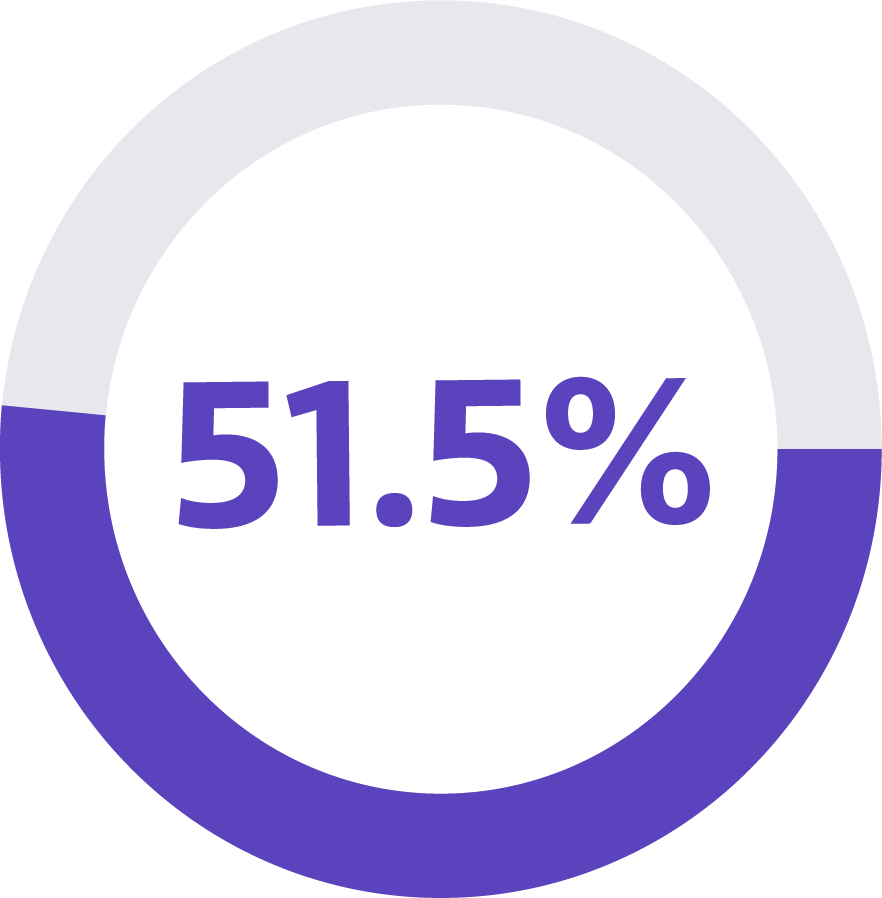
sensitivity*
This means that the Galleri test reported a “Cancer Signal Detected” result in approximately 52 individuals out of 100 who actually had cancer.1
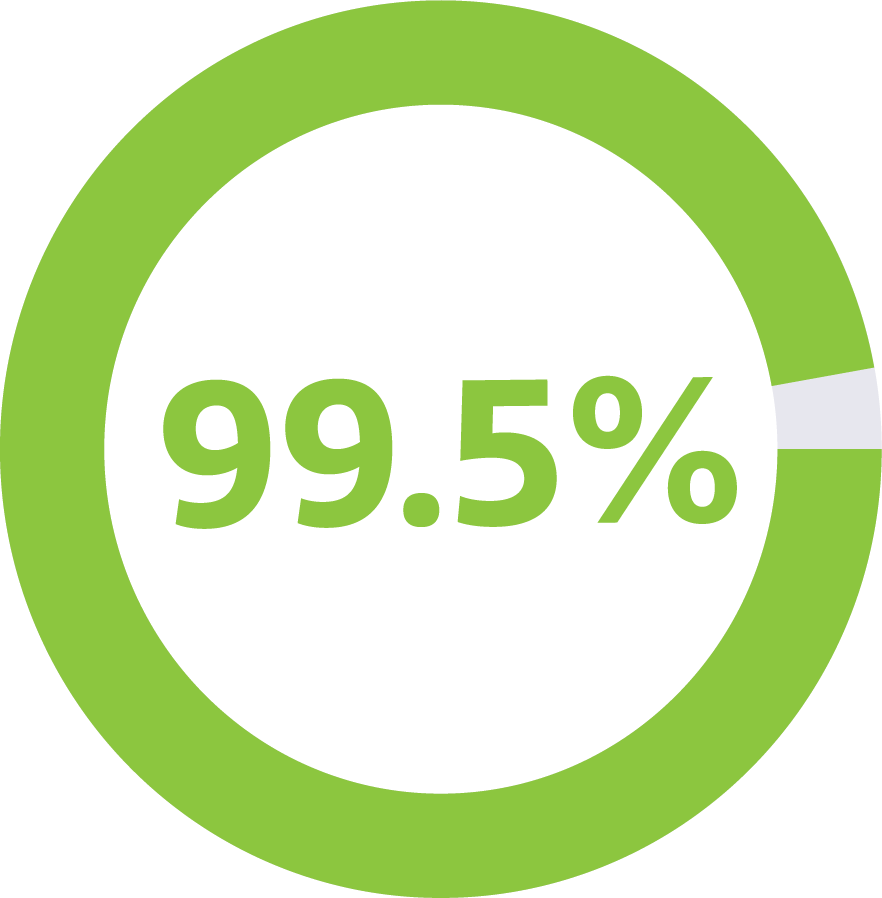
specificity
This means that the Galleri test reported a “Cancer Signal Not Detected” result in approximately 99 individuals out of 100 who do not actually have cancer.1
false positive rate
This means that the Galleri test reported a “Cancer Signal Detected” result 1 in every 200 tests run in individuals who did NOT actually have cancer.1
Cancer Signal Origin Accuracy
This means when a cancer signal was detected in an individual who actually had cancer, the first Cancer Signal Origin was correctly predicted in 89 of 100 times.1
*Sensitivity value is for all cancers and participants of all ages included in the CCGA3 subset.
Breakthrough Test Performance
sensitivity*
This means that the Galleri test reported a “Cancer Signal Detected” result in approximately 52 individuals out of 100 who actually had cancer.1

sensitivity*
This means that the Galleri test reported a “Cancer Signal Detected” result in approximately 52 individuals out of 100 who actually had cancer.1
specificity
This means that the Galleri test reported a “Cancer Signal Not Detected” result in approximately 99 individuals out of 100 who do not actually have cancer.1
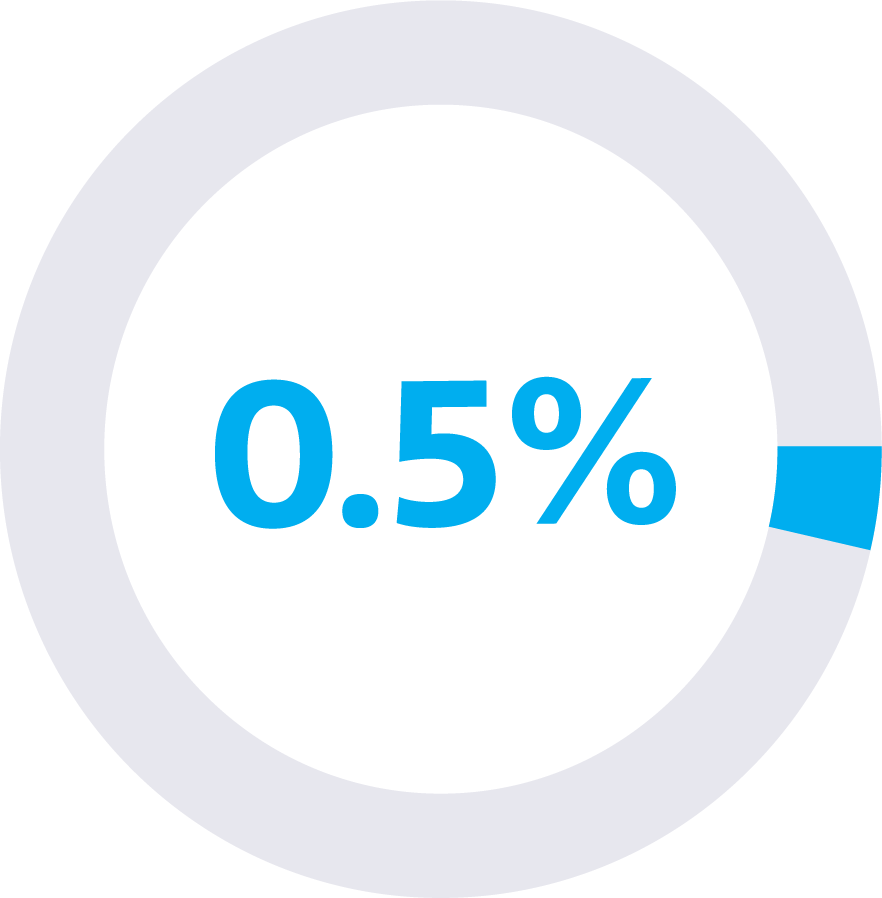
false positive rate
This means that the Galleri test reported a “Cancer Signal Detected” result 1 in every 200 tests run in individuals who did NOT actually have cancer.1
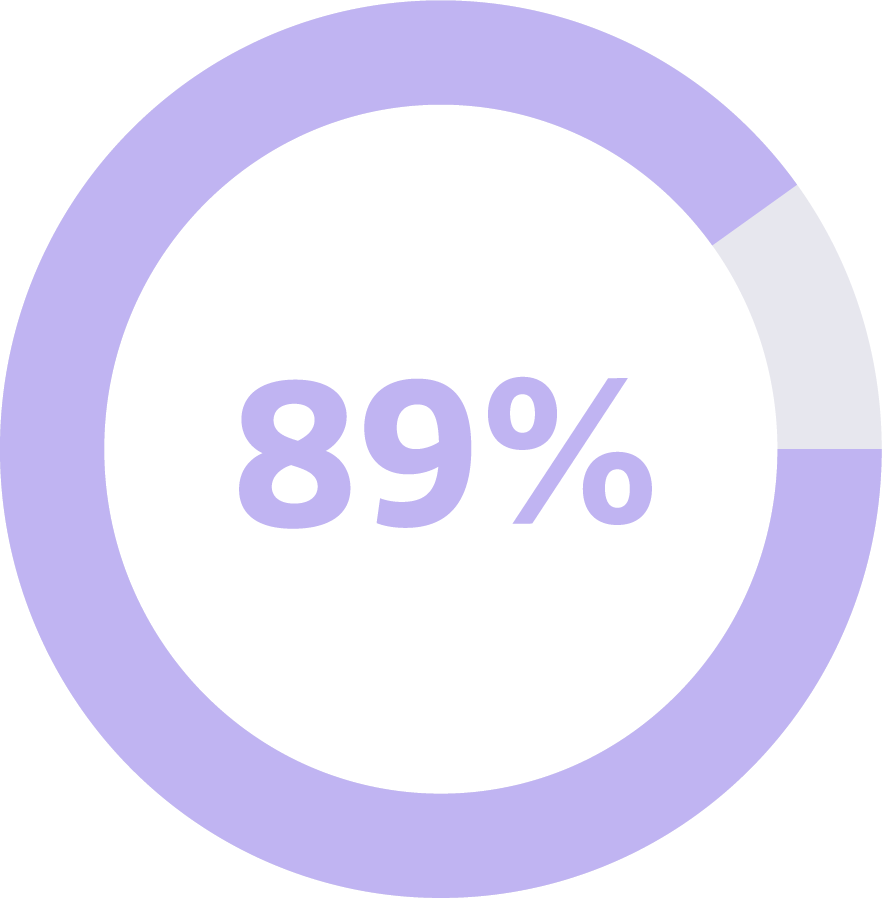
Cancer Signal Origin Accuracy
This means when a cancer signal was detected in an individual who actually had cancer, the first Cancer Signal Origin was correctly predicted in 89 of 100 times.1
*Sensitivity value is for all cancers and participants of all ages included in the CCGA3 subset.
Have questions about the Galleri test?
Contact the providers below that correlate with your inquiry.
Have questions about the Galleri test?
Contact the providers below that correlate with your inquiry.
Frequently Asked Questions
Am I eligible for the Galleri test?
BETWEEN THE AGES OF 40-49?
Certain risk factors may put you at an elevated risk for developing cancer. The Galleri test may be right for you if you meet one or more of the following criteria:
- You are a smoker or have quit smoking in the last 10 years
- You have a first degree relative (parent, child and/or full sibling) who has had cancer
- You have tested positive for HIV
- You have received an organ transplant
50 OR OLDER?
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those aged 50 or older. The risk of cancer increases as we age, so individuals 50 or older are eligible for the Galleri Test.
CURRENTLY, THE FOLLOWING INDIVIDUALS ARE NOT ELIGIBLE FOR THE GALLERI TEST:
- You have been diagnosed or treated for cancer within the past three years
- This excludes basal or squamous cell carcinomas of the skin
- This also excludes hormonal therapy (e.g., Tamoxifen, Arimidex, Aromasin) for breast cancer; you need to be 3 years past surgery, chemotherapy and/or radiotherapy but can still be taking hormonal therapy
- You are 39 years of age or younger
- You are currently pregnant
- You live outside of the United States; Genome Medical is not able to order testing for people who reside outside the US
How do I order the test?
The Galleri test must be ordered through a healthcare provider. If you’re ready to request the test, first you’ll be asked to answer a few health questions, and then a Genome Medical physician will review your information and determine if the test is right for you.
Is a consultation with a Genome Medical clinician required to get the test?
You can request the test without a pre-test consultation. However, a Genome Medical physician must review your provided health information to determine if the test is right for you.
Can I order the test through my personal healthcare provider?
In order to receive the test and genetic counseling at no cost, you must request the test through the Novartis and Genome Medical employer program.
Is the test available outside of the United States?
The Galleri test is not yet commercially available outside of the United States or in US territories.
What is the Galleri test?
The Galleri test is a multi-cancer early detection test that detects more than 50 types of cancer, many of which are not commonly screened for today, through a simple blood draw. The Galleri test does not diagnose cancer and not all cancers can be detected by the test.
Which cancers does the Galleri test detect?
In a large-scale clinical evaluation sub-study of more than 4,000 people, the Galleri test detected more than 50 cancer types across all stages. The test identified ~17% of individuals with stage I cancer, 40% with stage II cancer, 77% of individuals with stage III cancer and 90% of stage IV cancers.
How can the Galleri test detect cancer in the blood?
The Galleri test looks for signals present in the blood that may be associated with cancer at the time of your blood draw. If a cancer signal is detected, the results can point to where in the body the cancer signal is coming from with high accuracy to help your healthcare provider guide next steps.
Why is the Galleri test recommended to be used along with other cancer screening tests?
The Galleri test screens for more than 50 cancers; however, not all cancers can be detected by Galleri. Recommended routine cancer screening tests, such as a colonoscopy or mammogram are commonly used and have been shown to reduce cancer deaths. Galleri is intended to be used in addition to, not to replace, these tests and can help maximize the benefits of early cancer detection. Missing recommended screening or ignoring symptoms could lead to a delayed diagnosis of cancer. Ask Genome Medical about which cancer screening tests are right for you.
What is the false positive rate of the Galleri test?
The Galleri test has a low false-positive rate of 0.5% (detecting a cancer signal when no cancer is present). This means that in approximately 200 people tested, only 1 person would be expected to receive a false positive result.
What data supports the Galleri test?
The Galleri test development was supported by what is believed to be the largest clinical study program in genomic medicine, with over 20,000 participants at more than 140 clinical study sites, including the Mayo Clinic, Dana-Farber Cancer Institute, Cleveland Clinic, Sutter Health, Oregon Health & Science University, Intermountain Healthcare, and U.S. Oncology Research.
How quickly will I receive my results?
It takes about 2 weeks after your blood draw to receive your test results.
At that time, Genome Medical will contact you via email or phone to schedule an optional post-test consultation with a Genome Medical clinician to review your test results and discuss the next steps. Once you receive your results, it’s important to share your results and clinical action plan (which will be provided by Genome Medical) with your primary care physician.
What does it mean if I receive a “Cancer Signal Not Detected” result?
This means that no cancer signal was found. However, not all cancers can be detected by the Galleri test, and not all cancers may be detected in the blood. Continue with all routine screening tests that your healthcare provider recommends. Missing routine screenings or ignoring symptoms can lead to a delayed diagnosis of cancer.
What does it mean if I receive a “Cancer Signal Detected” result?
This means that there is a suspicion of cancer, but not a diagnosis of cancer. The Genome Medical provider that ordered your test will share this result with you and what it may mean.
The Galleri test can point to where in the body the cancer is coming from with high accuracy to help your healthcare team guide next steps. Genome Medical will work with your primary care provider and Memorial Sloan Kettering Cancer Center (MSK) to guide you through the evaluation and work-up. Novartis has a partnership with MSK to provide access to cancer care and support for Novartis employees.
Genome Medical can share your results and clinical action plan with your primary care provider and other providers if requested.
Why are additional tests needed when the Galleri test detects a cancer signal?
Galleri is a screening test that detects cancer signals in blood. When a signal is detected, the Galleri test also indicates where in the body the signal is coming from with high accuracy.
The Galleri test is not a diagnostic test to confirm cancer status. Additional tests ordered by your primary healthcare provider are needed to confirm if cancer is present which may include blood work or imaging.
Can you share my Galleri test result with one of my healthcare providers?
Yes. You can request Genome Medical to share your test results and clinical action plan with any providers of your choosing.
Can I discuss my results with a Genome Medical healthcare provider?
Yes. You have the option to speak with a Genome Medical provider before and after the test is ordered.
I have a cancer signal detected, where can I receive further diagnostics and cancer care?
If you receive Galleri test results with a cancer signal detected, Genome Medical will work with you, your primary care provider and MSK Direct, a cancer benefits solution for Novartis employees to confirm if cancer is present. MSK Direct gives you and your family dedicated access to exceptional cancer care, support, and expert resources from the world’s leading specialists at Memorial Sloan Kettering Cancer Center (MSK).
Your healthcare team will assist you in confirming a diagnosis by using your recent Galleri test result, creating a treatment plan and providing treatment, if needed.
If you have questions about MSK Direct, you may contact their dedicated phone line for Novartis employees at 844-473-3191, or visit mskcc.org/Novartis.
I submitted my test request. What happens next?
After you request the test, you will be asked to answer a few short health history questions. A healthcare provider from Genome Medical will review your request and assess your eligibility. If approved, the test will be ordered and sent to GRAIL within 1-2 business days. GRAIL will email you your completed Test Requisition Form and you will be shipped a Galleri specimen collection kit that you will bring to your blood draw appointment.
Once you receive your collection kit in the mail, you can schedule a blood draw appointment at the Primary Care at Novartis Morristown location. To schedule, please call 862-778-7960.
Please do not open your collection kit – this should remain unopened when you take it to the lab.
Where can I get my blood drawn for the Galleri test?
If your Galleri test request is approved, you can schedule a blood draw appointment at the Primary Care at Novartis Morristown location at no additional cost to you.
Please wait to schedule your blood draw appointment until you’ve received your collection kit in the mail. Please do not open your collection kit – this should remain unopened when you take it to the lab. To schedule, please call 862-778-7960.
Primary Care at Novartis
101 Madison Avenue, Suite 220
Morristown, NJ, 07960
What should I bring to my blood draw appointment?
Bring your unopened Galleri collection kit and your completed and printed ‘Test Requisition Form’ to your blood draw appointment.
You will receive these items if your test is approved.
- A collection kit will be mailed to you within one week after your request is approved. Please do not open the collection kit.
- A completed Test Requisition Form will be emailed to you from GRAIL. Remember to print the form for your appointment.
If you did not receive an email and believe you should have, please contact GRAIL Customer Services at 833-694-2553 or customerservice@grail.com.
What is my Test Requisition Form and where can I find it?
The Test Requisition Form is the ordering form the provider completes to order the test for you. You will receive this document via an order confirmation email from GRAIL if the provider orders your test – typically within 1-2 business days from when you requested the test.
How do I return the Galleri collection kit after my blood draw?
The lab technician who draws your blood will pack and ship your sample back to the GRAIL laboratory for processing.
Do I need to fast prior to the blood draw?
No preparation or fasting is required.
Some lab technicians may ask if you have fasted, but rest assured this is not required for the Galleri test.
How much blood is needed for the Galleri test?
Approximately 1.5 tablespoons (or about 20 mL) of blood in two tubes typically from a vein in your arm.
How much does the Galleri test cost?
Clinical eligibility must be met in order for Genome Medical to order the test. If clinical eligibility is met, the Galleri multi-cancer early detection test, and genetic services, are available as an annual benefit to you at no cost through the Genome Medical and Novartis Employer Program.
The test is currently not available outside of the US.
How will Novartis protect confidentiality as well as my health information and the health of my family member(s), including the Galleri test information?
Novartis takes patient privacy seriously. Novartis, Genome Medical and the GRAIL Laboratory will maintain the health information in compliance with all applicable medical privacy laws, including HIPAA, GINA, etc. Neither Genome Medical nor GRAIL will not share any health information with Novartis. Use of the Genomics Resource Center is confidential.
Frequently Asked Questions
Am I eligible for the Galleri test?
BETWEEN THE AGES OF 40-49?
Certain risk factors may put you at an elevated risk for developing cancer. The Galleri test may be right for you if you meet one or more of the following criteria:
- You are a smoker or have quit smoking in the last 10 years
- You have a first degree relative (parent, child and/or full sibling) who has had cancer
- You have tested positive for HIV
- You have received an organ transplant
50 OR OLDER?
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those aged 50 or older. The risk of cancer increases as we age, so individuals 50 or older are eligible for the Galleri Test.
CURRENTLY, THE FOLLOWING INDIVIDUALS ARE NOT ELIGIBLE FOR THE GALLERI TEST:
- You have been diagnosed or treated for cancer within the past three years
- This excludes basal or squamous cell carcinomas of the skin
- This also excludes hormonal therapy (e.g., Tamoxifen, Arimidex, Aromasin) for breast cancer; you need to be 3 years past surgery, chemotherapy and/or radiotherapy but can still be taking hormonal therapy
- You are 39 years of age or younger
- You are currently pregnant
- You live outside of the United States; Genome Medical is not able to order testing for people who reside outside the US
How do I order the test?
The Galleri test must be ordered through a healthcare provider. If you’re ready to request the test, first you’ll be asked to answer a few health questions, and then a Genome Medical physician will review your information and determine if the test is right for you.
Is a consultation with a Genome Medical clinician required to get the test?
You can request the test without a pre-test consultation. However, a Genome Medical physician must review your provided health information to determine if the test is right for you.
Can I order the test through my personal healthcare provider?
In order to receive the test and genetic counseling at no cost, you must request the test through the Novartis and Genome Medical employer program.
Is the test available outside of the United States?
The Galleri test is not yet commercially available outside of the United States or in US territories.
What is the Galleri test?
The Galleri test is a multi-cancer early detection test that detects more than 50 types of cancer, many of which are not commonly screened for today, through a simple blood draw. The Galleri test does not diagnose cancer and not all cancers can be detected by the test.
Which cancers does the Galleri test detect?
In a large-scale clinical evaluation sub-study of more than 4,000 people, the Galleri test detected more than 50 cancer types across all stages. The test identified ~17% of individuals with stage I cancer, 40% with stage II cancer, 77% of individuals with stage III cancer and 90% of stage IV cancers.
How can the Galleri test detect cancer in the blood?
The Galleri test looks for signals present in the blood that may be associated with cancer at the time of your blood draw. If a cancer signal is detected, the results can point to where in the body the cancer signal is coming from with high accuracy to help your healthcare provider guide next steps.
Why is the Galleri test recommended to be used along with other cancer screening tests?
The Galleri test screens for more than 50 cancers; however, not all cancers can be detected by Galleri. Recommended routine cancer screening tests, such as a colonoscopy or mammogram are commonly used and have been shown to reduce cancer deaths. Galleri is intended to be used in addition to, not to replace, these tests and can help maximize the benefits of early cancer detection. Missing recommended screening or ignoring symptoms could lead to a delayed diagnosis of cancer. Ask Genome Medical about which cancer screening tests are right for you.
What is the false positive rate of the Galleri test?
The Galleri test has a low false-positive rate of 0.5% (detecting a cancer signal when no cancer is present). This means that in approximately 200 people tested, only 1 person would be expected to receive a false positive result.
What data supports the Galleri test?
The Galleri test development was supported by what is believed to be the largest clinical study program in genomic medicine, with over 20,000 participants at more than 140 clinical study sites, including the Mayo Clinic, Dana-Farber Cancer Institute, Cleveland Clinic, Sutter Health, Oregon Health & Science University, Intermountain Healthcare, and U.S. Oncology Research.
How quickly will I receive my results?
It takes about 2 weeks after your blood draw to receive your test results.
At that time, Genome Medical will contact you via email or phone to schedule an optional post-test consultation with a Genome Medical clinician to review your test results and discuss the next steps. Once you receive your results, it’s important to share your results and clinical action plan (which will be provided by Genome Medical) with your primary care physician.
What does it mean if I receive a “Cancer Signal Not Detected” result?
This means that no cancer signal was found. However, not all cancers can be detected by the Galleri test, and not all cancers may be detected in the blood. Continue with all routine screening tests that your healthcare provider recommends. Missing routine screenings or ignoring symptoms can lead to a delayed diagnosis of cancer.
What does it mean if I receive a “Cancer Signal Detected” result?
This means that there is a suspicion of cancer, but not a diagnosis of cancer. The Genome Medical provider that ordered your test will share this result with you and what it may mean.
The Galleri test can point to where in the body the cancer is coming from with high accuracy to help your healthcare team guide next steps. Genome Medical will work with your primary care provider and Memorial Sloan Kettering Cancer Center (MSK) to guide you through the evaluation and work-up. Novartis has a partnership with MSK to provide access to cancer care and support for Novartis employees.
Genome Medical can share your results and clinical action plan with your primary care provider and other providers if requested.
Why are additional tests needed when the Galleri test detects a cancer signal?
Galleri is a screening test that detects cancer signals in blood. When a signal is detected, the Galleri test also indicates where in the body the signal is coming from with high accuracy.
The Galleri test is not a diagnostic test to confirm cancer status. Additional tests ordered by your primary healthcare provider are needed to confirm if cancer is present which may include blood work or imaging.
Can you share my Galleri test result with one of my healthcare providers?
Yes. You can request Genome Medical to share your test results and clinical action plan with any providers of your choosing.
Can I discuss my results with a Genome Medical healthcare provider?
Yes. You have the option to speak with a Genome Medical provider before and after the test is ordered.
I have a cancer signal detected, where can I receive further diagnostics and cancer care?
If you receive Galleri test results with a cancer signal detected, Genome Medical will work with you, your primary care provider and MSK Direct, a cancer benefits solution for Novartis employees to confirm if cancer is present. MSK Direct gives you and your family dedicated access to exceptional cancer care, support, and expert resources from the world’s leading specialists at Memorial Sloan Kettering Cancer Center (MSK).
Your healthcare team will assist you in confirming a diagnosis by using your recent Galleri test result, creating a treatment plan and providing treatment, if needed.
If you have questions about MSK Direct, you may contact their dedicated phone line for Novartis employees at 844-473-3191, or visit mskcc.org/Novartis.
I submitted my test request. What happens next?
After you request the test, you will be asked to answer a few short health history questions. A healthcare provider from Genome Medical will review your request and assess your eligibility. If approved, the test will be ordered and sent to GRAIL within 1-2 business days. GRAIL will email you your completed Test Requisition Form and you will be shipped a Galleri specimen collection kit that you will bring to your blood draw appointment.
Once you receive your collection kit in the mail, you can schedule a blood draw appointment at the Primary Care at Novartis Morristown location. To schedule, please call 862-778-7960.
Please do not open your collection kit – this should remain unopened when you take it to the lab.
Where can I get my blood drawn for the Galleri test?
If your Galleri test request is approved, you can schedule a blood draw appointment at the Primary Care at Novartis Morristown location at no additional cost to you.
Please wait to schedule your blood draw appointment until you’ve received your collection kit in the mail. Please do not open your collection kit – this should remain unopened when you take it to the lab. To schedule, please call 862-778-7960.
Primary Care at Novartis
101 Madison Avenue, Suite 220
Morristown, NJ, 07960
What should I bring to my blood draw appointment?
Bring your unopened Galleri collection kit and your completed and printed ‘Test Requisition Form’ to your blood draw appointment.
You will receive these items if your test is approved.
- A collection kit will be mailed to you within one week after your request is approved. Please do not open the collection kit.
- A completed Test Requisition Form will be emailed to you from GRAIL. Remember to print the form for your appointment.
If you did not receive an email and believe you should have, please contact GRAIL Customer Services at 833-694-2553 or customerservice@grail.com.
What is my Test Requisition Form and where can I find it?
The Test Requisition Form is the ordering form the provider completes to order the test for you. You will receive this document via an order confirmation email from GRAIL if the provider orders your test – typically within 1-2 business days from when you requested the test.
How do I return the Galleri collection kit after my blood draw?
The lab technician who draws your blood will pack and ship your sample back to the GRAIL laboratory for processing.
Do I need to fast prior to the blood draw?
No preparation or fasting is required.
Some lab technicians may ask if you have fasted, but rest assured this is not required for the Galleri test.
How much blood is needed for the Galleri test?
Approximately 1.5 tablespoons (or about 20 mL) of blood in two tubes typically from a vein in your arm.
How much does the Galleri test cost?
Clinical eligibility must be met in order for Genome Medical to order the test. If clinical eligibility is met, the Galleri multi-cancer early detection test, and genetic services, are available as an annual benefit to you at no cost through the Genome Medical and Novartis Employer Program.
The test is currently not available outside of the US.
How will Novartis protect confidentiality as well as my health information and the health of my family member(s), including the Galleri test information?
Novartis takes patient privacy seriously. Novartis, Genome Medical and the GRAIL Laboratory will maintain the health information in compliance with all applicable medical privacy laws, including HIPAA, GINA, etc. Neither Genome Medical nor GRAIL will not share any health information with Novartis. Use of the Genomics Resource Center is confidential.
Important Safety Information
The Galleri® test is recommended for use in adults with an elevated risk for cancer, such as those aged 50 or older. The Galleri test does not detect all cancers and should be used in addition to routine cancer screening tests recommended by a health care provider. Galleri is intended to detect cancer signals and predict where in the body the cancer signal is located.
Results should be interpreted by a health care provider in the context of medical history, clinical signs and symptoms. A test result of “Cancer Signal Not Detected” does not rule out cancer. A test result of “Cancer Signal Detected” requires confirmatory diagnostic evaluation by medically established procedures (e.g. imaging) to confirm cancer.
If cancer is not confirmed with further testing, it could mean that cancer is not present or testing was insufficient to detect cancer, including due to the cancer being located in a different part of the body. False-positive (a cancer signal detected when cancer is not present) and false-negative (a cancer signal not detected when cancer is present) test results do occur. Rx only.
Laboratory/Test Information
GRAIL’s clinical laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and accredited by the College of American Pathologists (CAP). The Galleri test was developed, and its performance characteristics were determined by GRAIL.
References and Notes
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021;32(9):1167-1177.
- Amin MB, et al. (Eds). American Joint Committee of Cancer (AJCC) Cancer Staging Manual (8th edition).
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence — SEER 18 Regs Research Data. Nov 2018 Sub. Includes persons aged 50 – 79 diagnosed 2006 – 2015. “Early/ Localized” includes invasive localized tumors that have not spread beyond organ of origin. “Late/ Metastasized” includes invasive cancers that have metastasized beyond the organ of origin to other parts of the body.
- Modeled detection extrapolated to 2020 US population ages 50 – 79. Screening includes methods with United States Preventive Services Task Force (USPSTF) A, B, or C rating (breast, colon, cervical, prostate, and lung), and assumes screening is available for all prostate, breast, cervical, and colorectal cancer cases and 33% of lung cancer cases (based on estimated proportion of lung cancers that occur in screen-eligible individuals older than 40 years).
- Data on file from Surveillance, Epidemiology, and End Results (SEER) 18 Regs Research Data, Nov 2017 Submission. Includes persons aged 50 – 79. Estimated deaths per year in 2020 from American Cancer Society Cancer Facts and Figures 2020. Available at: www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf
- Assumes screening is available for all prostate, breast, cervical, and colorectal cancer cases and 43% of lung cancer cases (based on estimated proportion of lung cancers that occur in screen-eligible individuals older than 40 years) Source: Estimated deaths per year in 2021 from American Cancer Society Cancer Facts and Figures 2021. Available at: http://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf. Data on file GA-2021-0065
- Noone AM, Howlader N, Krapcho M, et al. (eds). SEER Cancer Statistics Review, 1975 – 2015, National Cancer Institute, Bethesda, MD, http://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER website April 2018.
- Screening includes methods with United States Preventative Services Task Force (USPSTF) A, B, or C rating (breast, colon, cervical, prostate, and lung).
Important Safety Information
The Galleri® test is recommended for use in adults with an elevated risk for cancer, such as those aged 50 or older. The Galleri test does not detect all cancers and should be used in addition to routine cancer screening tests recommended by a health care provider. Galleri is intended to detect cancer signals and predict where in the body the cancer signal is located.
Results should be interpreted by a health care provider in the context of medical history, clinical signs and symptoms. A test result of “Cancer Signal Not Detected” does not rule out cancer. A test result of “Cancer Signal Detected” requires confirmatory diagnostic evaluation by medically established procedures (e.g. imaging) to confirm cancer.
If cancer is not confirmed with further testing, it could mean that cancer is not present or testing was insufficient to detect cancer, including due to the cancer being located in a different part of the body. False-positive (a cancer signal detected when cancer is not present) and false-negative (a cancer signal not detected when cancer is present) test results do occur. Rx only.
Laboratory/Test Information
GRAIL’s clinical laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and accredited by the College of American Pathologists (CAP). The Galleri test was developed, and its performance characteristics were determined by GRAIL.
References and Notes
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021;32(9):1167-1177.
- Amin MB, et al. (Eds). American Joint Committee of Cancer (AJCC) Cancer Staging Manual (8th edition).
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence — SEER 18 Regs Research Data. Nov 2018 Sub. Includes persons aged 50 – 79 diagnosed 2006 – 2015. “Early/ Localized” includes invasive localized tumors that have not spread beyond organ of origin. “Late/ Metastasized” includes invasive cancers that have metastasized beyond the organ of origin to other parts of the body.
- Modeled detection extrapolated to 2020 US population ages 50 – 79. Screening includes methods with United States Preventive Services Task Force (USPSTF) A, B, or C rating (breast, colon, cervical, prostate, and lung), and assumes screening is available for all prostate, breast, cervical, and colorectal cancer cases and 33% of lung cancer cases (based on estimated proportion of lung cancers that occur in screen-eligible individuals older than 40 years).
- Data on file from Surveillance, Epidemiology, and End Results (SEER) 18 Regs Research Data, Nov 2017 Submission. Includes persons aged 50 – 79. Estimated deaths per year in 2020 from American Cancer Society Cancer Facts and Figures 2020. Available at: www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf
- Assumes screening is available for all prostate, breast, cervical, and colorectal cancer cases and 43% of lung cancer cases (based on estimated proportion of lung cancers that occur in screen-eligible individuals older than 40 years) Source: Estimated deaths per year in 2021 from American Cancer Society Cancer Facts and Figures 2021. Available at: http://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf. Data on file GA-2021-0065
- Noone AM, Howlader N, Krapcho M, et al. (eds). SEER Cancer Statistics Review, 1975 – 2015, National Cancer Institute, Bethesda, MD, http://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER website April 2018.
- Screening includes methods with United States Preventative Services Task Force (USPSTF) A, B, or C rating (breast, colon, cervical, prostate, and lung).