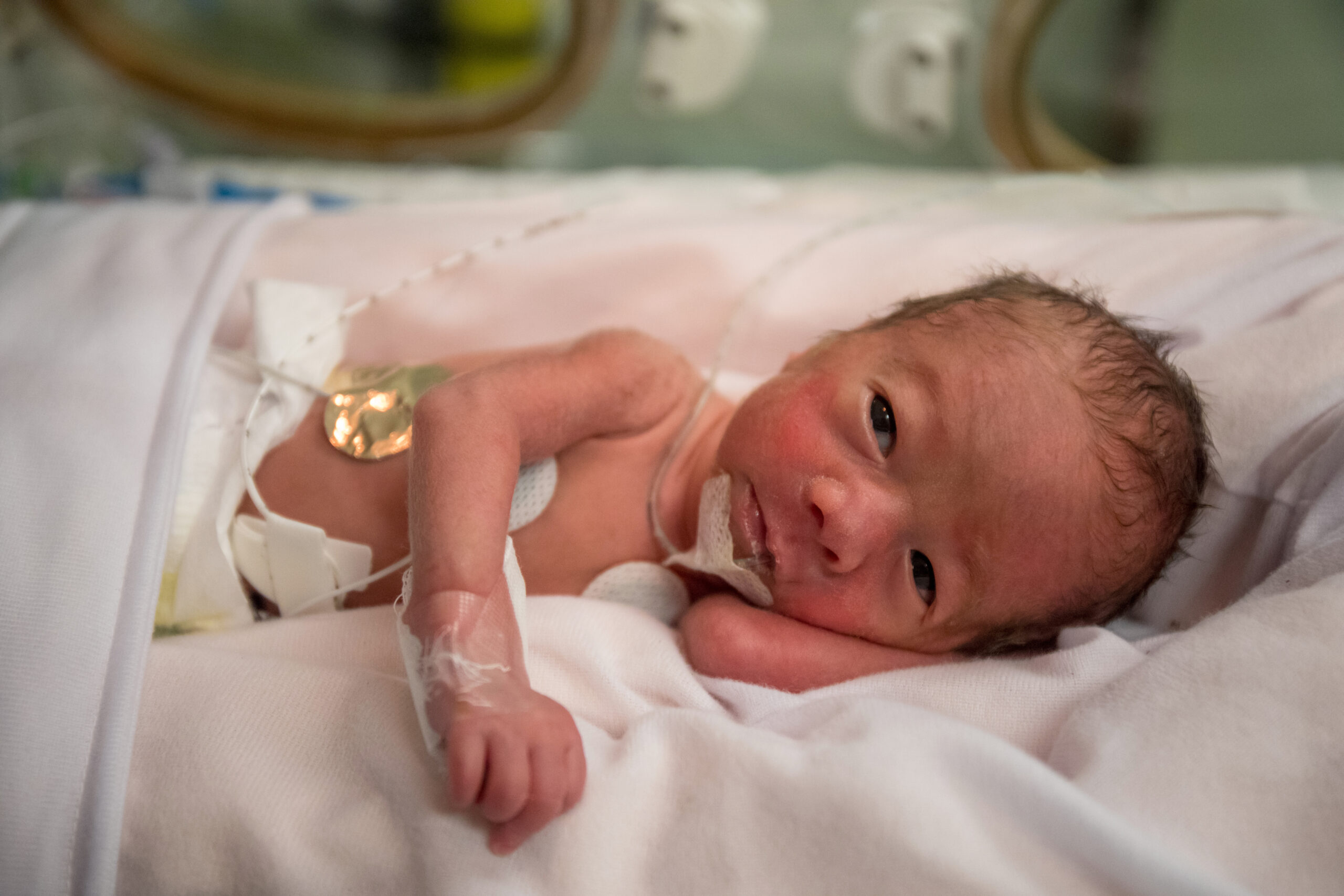
Margie and Chris were elated when their daughter Hazel was born. They gazed at her new face and recognized themselves, maybe Chris’s grandma, a touch of Margie’s cousin. They vowed then and there to protect her and do whatever they could to make her way in the world as easy as possible. But in the midst of their joy they noticed a pause in Hazel’s breathing and a jerk of her left arm. The doctors dismissed it at first as “just what babies do.” But the pauses lengthened, followed by more jerking, this time sustained and rhythmic. Suddenly the little bundle went limp in Margie’s arms. Hazel had stopped breathing, her lips turned blue. Nurses and doctors rushed into the room and whisked Hazel away for blood tests, monitoring, interventions and intensive care.
Hazel’s parents were adrift. What’s happening? What’s going on? The doctors suspected she was having seizures, but they didn’t know why. They called specialists and offered ideas, but no diagnosis. They ordered studies, some of them invasive, results for which took weeks to get back and one by one they returned without answers. The first scary hours stretched into days and weeks and became a blurry jumble of medical jargon and daily reports: machines, drugs, nutrition, complex details, but still no one could say why. Why is this happening to Hazel?
Newborn Sequencing Can Provide an Early Diagnosis for Sick Babies
For many acutely ill newborns, there may be an answer hiding in plain sight. Approximately 36 percent of babies who are admitted to the neonatal intensive care unit (NICU) have an identifiable genetic disorder, a genetic mutation (or mutations) that causes or predisposes to serious illness. There are over 7000 genetic disorders currently identified, a number that is likely to swell in the coming years, making it nearly impossible for any clinician to keep up with them, let alone recognize some of them immediately after birth.
Luckily, we now have a critical tool in our arsenal – a comprehensive genetic test from a blood draw or cheek swab. What started with DNA’s discovery in 1953 and continued through the expensive and international effort to draft the whole human genome in 2003, is now a test that any clinician in the U.S. can order when they suspect a genetic disorder. It is now possible to analyze an entire human genome in a week for less than $1000. Driving down the cost and turn around time for clinical genome tests will be a potential game changer for infants like Hazel.
It’s likely that Whole Genome Sequencing (WGS) will become an important tool for precision medicine in myriad clinical contexts. Only one such scenario is the NICU where a fast, accurate diagnosis is important. Study after study has now shown that genome sequencing not only gives parents and clinicians a diagnosis (and some degree of clarity) but can also lead to important changes in a baby’s medical management. In Hazel’s case, she was subjected to three long months of intensive care and management decisions based on clinical suspicions before her genetic diagnosis was finally discovered. As it turns out, she had an ultra-rare mitochondrial disorder and while there was no cure, there were treatment strategies based on her unique biochemistry that could have – perhaps should have – been tried earlier in her course.
Early Diagnosis Benefits the Patient and Saves Money
What if we’d known from the beginning what the culprit was? Would it have changed the trajectory of Hazel’s course? Many medical professionals are quick to point out that a timely, accurate genetic diagnosis oftentimes directly results in changes in care management and subsequent health outcomes. Moreover, doctors of babies who have had their genomes sequenced overwhelmingly find it useful, regardless of the result.
The FDA continues to approve new and potentially curative therapies for genetic disorders. This is important: If there are specific treatments available for a genetic disorder – neonatal onset or otherwise – then it stands to reason (and holds up under scrutiny) that the earlier you diagnose, the quicker you can treat and secure the biggest benefit for your patient. Right now, there are ~650 genetic disorders with treatments associated with them.
It’s Time to Provide Access to Newborn Sequencing Across the Board
But knowing we should do something isn’t the same as doing it. There is evidence that healthcare systems are not integrating genetic and genomic testing early enough in a child’s NICU course, if at all. Outside the major academic centers, there are few pediatricians and hospitals who have access to rapid WGS to guide their diagnoses and tailor their management plans.
One of the major reasons for this apparent ‘know-do’ gap is the small and stagnant clinical genetics workforce that has historically diagnosed and cared for patients living with genetic disorders. There are fewer than 1,800 clinical geneticists and 4,200 genetic counselors working with patients in the field, compared to 209,000 primary care physicians and 48,000 emergency room doctors. In many clinics it can take six to 12 months to get an appointment with a genetics-trained provider and it still takes an average of five years to get an accurate diagnosis.
We have to do better. For starters, patients need access to WGS and parents want it. Clinicians who specialize in genetic disorders also want it and are all too familiar with the gaps in care that exist because it isn’t being appropriately utilized.
Removing Access as a Barrier to Care for NICU Patients
As genomic medicine continues to find its footing throughout health systems, including the NICU, reliable and timely access to high-quality clinical genetic services will remain a hurdle for some patients and their frontline providers. To meet this challenge head on, and to realize equitable access to the benefits of early genetic diagnosis, we need proven and promising tech-enabled service delivery models that can support providers on the frontline as well as their hospitals and health systems no matter where in the world they are located.
Through advancements in tech-enabled, scalable solutions, telegenetic counseling is drastically improving personalized medicine, care quality and outcomes for patients who have lacked access in the past. From test ordering and interpretation to provider decision support and genetic counseling, it is time to expand these services more broadly into standard clinical practice – in the NICU and beyond. Doing so will ensure that our society’s youngest, most vulnerable members, when they are sick, are given the best possible start in life and that their parents and healthcare providers are making those important early decisions with as much information as possible.
Find out more about pediatric medical genetics and genetic disorders in children.
About the Author: Brian Kirmse
Born in Miami, Dr. Kirmse is a board-certified pediatrician and clinical geneticist who has spent the last 20 years taking care of kids with rare disorders, in particular those with inherited metabolic diseases. He completed medical school at the University of Miami (2001), Pediatric Residency at the University of Florida (2004) and Clinical Genetics (2007) and Clinical Biochemical Genetics (2010) Fellowships at Mount Sinai Hospital in New York.

